Categories
Change Password!
Reset Password!


CKD-495 75 mg significantly improves gastric mucosal healing and symptom improvement in acute and chronic gastritis.
A recent phase II, multi-center, randomized, double-blind study has highlighted the potential of CKD-495, a novel drug derived from Cinnamomum cassia Presl, in effectively treating acute and chronic gastritis.
Gastritis remains a widespread gastrointestinal disorder, and the search for safer, faster-acting therapies continues to gain momentum as clinicians prioritize mucosal healing and symptom relief. CKD-495 has emerged as a promising investigational option with potential anti-inflammatory and mucosal-protective properties. This study by Su Hyun Park et al. aimed to assess the efficacy and safety of CKD-495 in patients with endoscopically confirmed gastritis.
Overall, 250 adults diagnosed with gastric mucosal erosion were randomly assigned to five treatment arms and received either CKD-495 (75 mg or 150 mg), rebamipide (100 mg), Artemisiae argyi folium extract (Stillen, 60 mg), or placebo for a 14-day course. The primary endpoint focused on endoscopic erosion improvement, while secondary endpoints examined erosion healing, symptom resolution, and reductions in edema, redness, and hemorrhagic lesions. Safety profiles and drug-related adverse events were monitored throughout the study.
The results showed that:
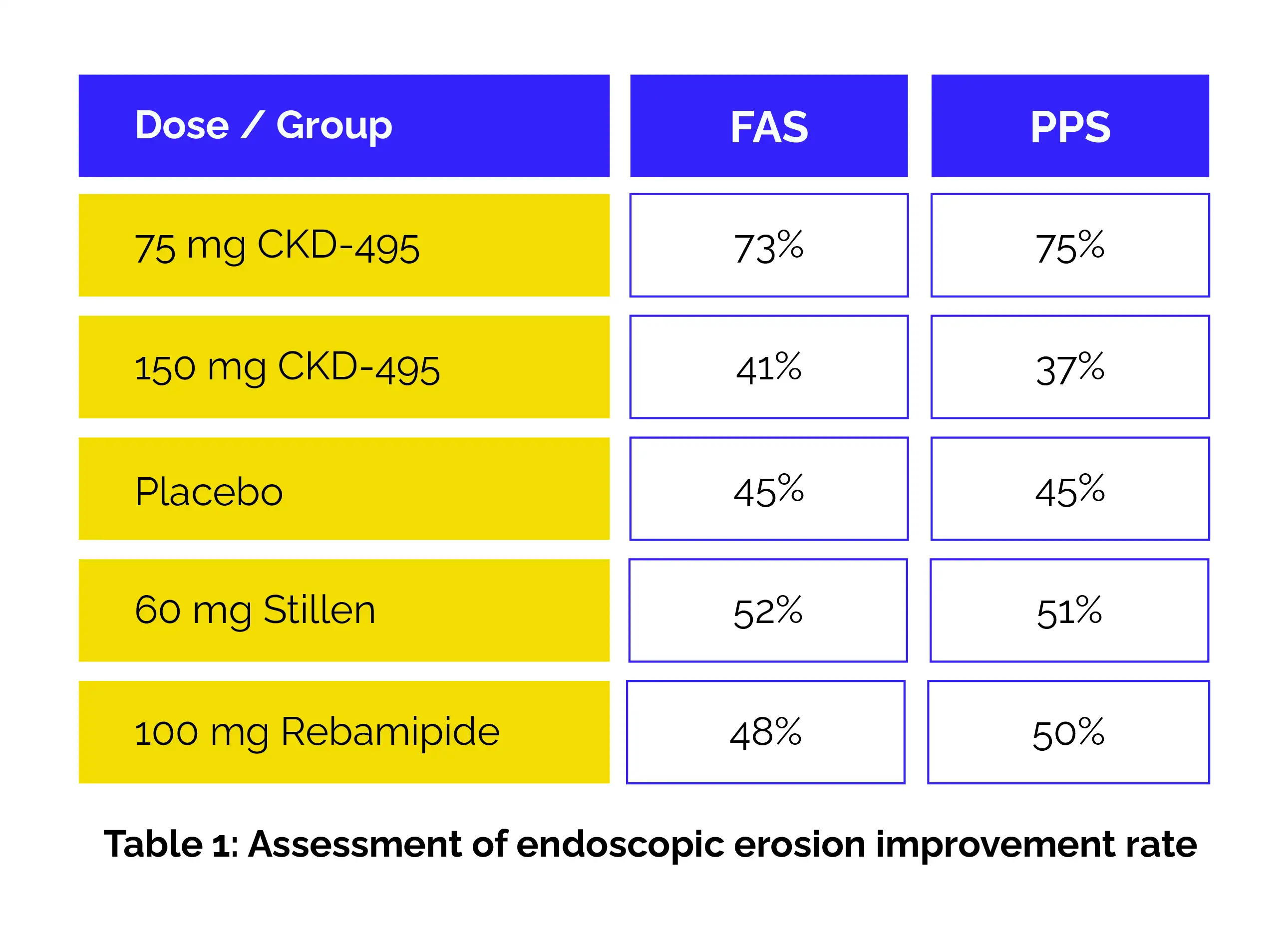
The gastric erosion cure rate was also highest with 75 mg CKD-495.
Interestingly, the 150 mg CKD-495 dose showed significant improvement in hemorrhagic erosions, suggesting a dose-dependent effect for specific gastric lesions.
CKD-495 was well-tolerated across all doses, with no serious adverse events or drug-related reactions reported.
The researchers concluded that CKD-495 at the 75 mg dose provided meaningful endoscopic and symptomatic improvements in acute and chronic gastritis without compromising safety. The treatment emerged as a well-tolerated and clinically effective option, supporting its potential advancement into larger confirmatory trials.
Canadian Journal of Gastroenterology and Hepatology
A Phase 2, Multi-Center, Randomized, Double-Blind, Parallel-Group Trial to Evaluate the Efficacy and Safety of CKD-495 in Patients With Acute and Chronic Gastritis
Su Hyun Park et al.
Comments (0)