Categories
Change Password!
Reset Password!


Autoimmune thyroid diseases represent the most common form of organ-specific autoimmunity, with Graves–Basedow disease (GBD) and Hashimoto’s thyroiditis constituting the two major clinical entities. GBD is driven by loss of immune tolerance to the thyrotropin receptor (TSHR), resulting in pathogenic TSHR antibodies that stimulate thyroid hormone overproduction.
Selenium supplementation boosts thyroid hormone levels (TSH, FT4, FT3), reduces thyroid autoantibodies (TPOAb, TgAb, TRAb), and enhances clinical outcomes—such as quality of life, reduced ocular involvement, and slower disease progression—in patients with GBD and GBD orbitopathy.
Autoimmune thyroid diseases represent the most common form of organ-specific autoimmunity, with Graves–Basedow disease (GBD) and Hashimoto’s thyroiditis constituting the two major clinical entities. GBD is driven by loss of immune tolerance to the thyrotropin receptor (TSHR), resulting in pathogenic TSHR antibodies that stimulate thyroid hormone overproduction. Clinically, GBD manifests with hyperthyroidism, diffuse goiter, ophthalmopathy, and, less frequently dermopathy. Global prevalence ranges from 0.5% to 2%, with a greater burden among females.
Nutritional influences, particularly micronutrient status, have been identified as key modifiers of autoimmune thyroid disease risk. Selenium is of particular relevance because the thyroid gland holds the highest selenium density per gram among human tissues. Selenium is incorporated into selenoproteins involved in antioxidant defense, thyroid hormone activation, and immune regulation. Deficiency has been linked to dysregulation of Th1/Th2 immune activity, reduced regulatory T lymphocytes function, increased oxidative stress, and impaired conversion of thyroxine (T4) to triiodothyronine (T3).
Multiple studies demonstrate that individuals with GBD exhibit lower serum selenium concentrations compared with healthy controls. This has led to the hypothesis that selenium supplementation may attenuate autoimmune activity, boost thyroid hormone balance, and reduce the severity of Graves’ orbitopathy. The therapeutic role of selenium is now of growing interest, but clinical practice guidelines remain inconsistent due to variation in trial methodologies and outcomes.
Objective
This scoping review sought to synthesize clinical evidence and evaluate selenium supplementation in GBD—with or without orbitopathy—across biochemical, immunological, and clinical outcome measures.
[1] Study design
The research was assessed by following the Preferred Reporting Items for systematic reviews and meta-analyses (PRISMA) and the modified version of the Population, Interventions, Comparators, and Outcomes (PICO) framework (Figure 1).
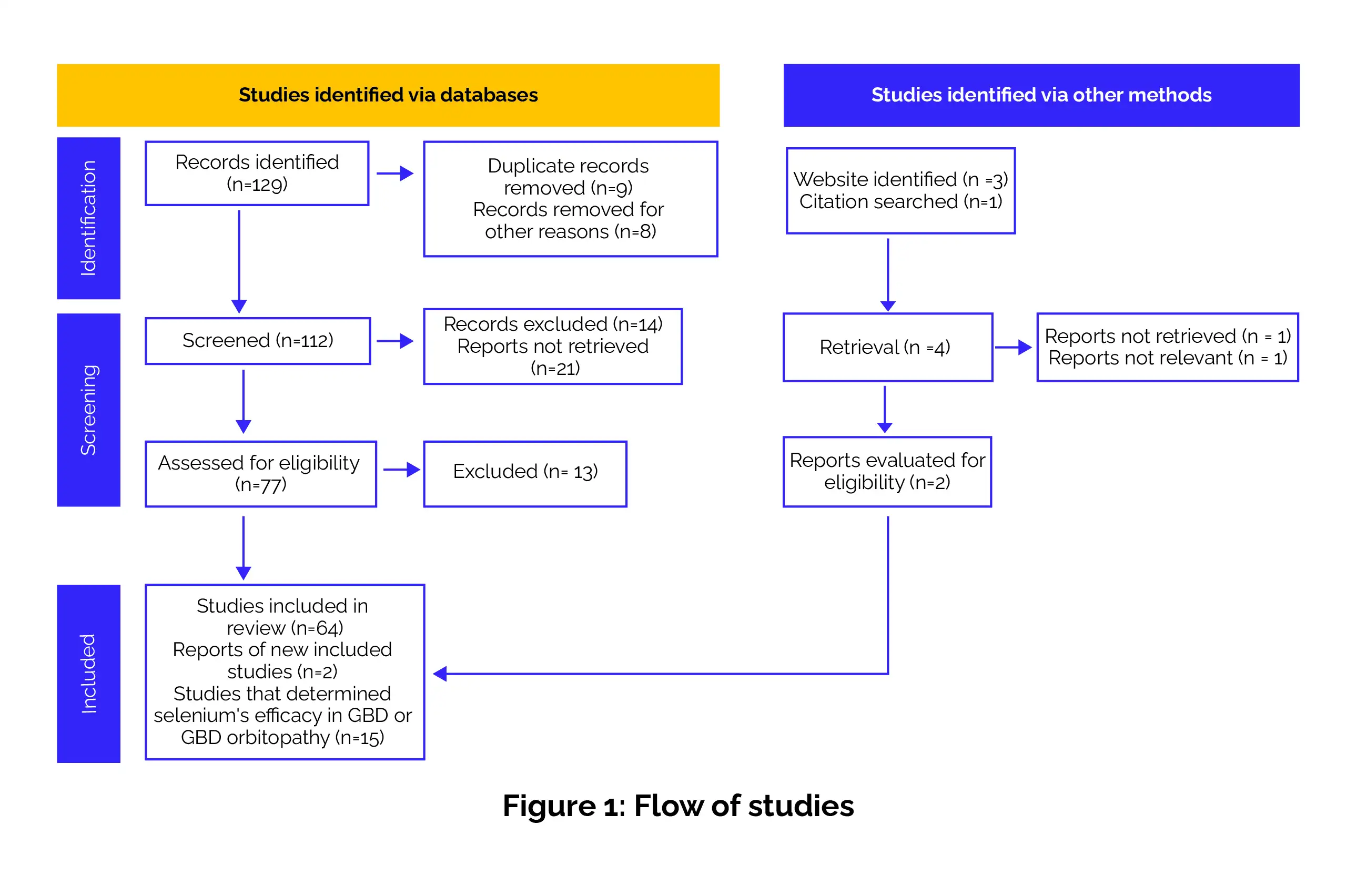
[2] Data sources and search strategy
A comprehensive search of Google Scholar, PubMed/Medline, Scopus, Biosis, ProQuest, and Web of Science was performed for English-language studies issued from January 2000 to March 2025. The search terms included combinations of:
[3] Screening and selection
Three independent reviewers screened titles and abstracts using the Rayyan web tool. Full texts were evaluated via the Joanna Briggs Institute (JBI) Critical Appraisal Checklist. Disagreements were resolved through consensus.
[4] Inclusion criteria
[5] Exclusion criteria
[6] Data extraction
Extracted data were recorded in a standardized Excel spreadsheet, including:
[7] Endpoints
Studies involving GBD patients mainly focused on biochemical and clinical markers before and after selenium supplementation. The key parameters evaluated included:
In contrast, studies involving patients with Graves-Basedow orbitopathy assessed selenium’s effect on ophthalmic and quality-of-life outcomes. The measures reported included:
[8] Statistical approach
Due to marked heterogeneity in study design, follow-up duration, and outcome definitions, no meta-analysis was performed. Results were synthesized descriptively.
[1] Study selection and characteristics
Sample size
Mostly <100 participants; predominantly women
Age
(a) GBD trials: Mean age 39.1 years (selenium group) vs. 39.7 years (control group)
(b) GBD orbitopathy trials: Mean age 42.2 years (selenium group) vs. 44.6 years (control group)
Follow-up duration
4 weeks – 5 years
[2] Selenium status and dosing
[3] Concomitant or previously utilized strategy for GBD
[4] Clinical outcomes following selenium intervention
This review synthesizes two decades of clinical evidence evaluating selenium supplementation in GBD and GBD orbitopathy. Across the included trials, selenium was associated with improvements in thyroid hormone profiles and reductions in autoimmune antibody titers. These findings align with mechanistic data showing that selenium-dependent enzymes regulate thyroid hormone activation, reduce oxidative stress, and support immune homeostasis.
The observed improvements in orbitopathy outcomes are biologically plausible given selenium’s antioxidant properties and its role in modulating fibroblast and cytokine activity in orbital tissues. The collective findings suggest that selenium may serve as a beneficial adjunct, particularly in patients residing in selenium-deficient regions or presenting with active orbitopathy. Future research must prioritize:
Additionally, research should also examine whether selenium has an additive or synergistic effect when combined with existing nonsurgical therapies for Graves orbitopathy—such as glucocorticoids, immunomodulators, biologics, radiation, or surgical intervention—and whether supplementation could minimize the need for more intensive treatments.
Selenium supplementation delivers consistent improvements in GBD and associated orbitopathy, enhancing thyroid hormone regulation and lowering autoimmune antibody levels. Patients with orbitopathy additionally experience reduced ocular inflammation and symptomatic relief. Overall, evidence supports selenium as a valuable adjunct to standard therapy, especially in individuals with documented selenium deficiency.
Medical Sciences
Effectiveness of Selenium Supplementation in the Treatment of Graves–Basedow Disease: A Scoping Review
Hernando Vargas-Uricoechea et al.
Comments (0)