Categories
Change Password!
Reset Password!


This phase III trial investigated tegoprazan's efficacy and safety as part of the first-line triple therapy regimen for Helicobacter pylori (H. pylori) eradication.
Tegoprazan-based triple therapy shows similar efficacy and safety to PPI-based triple therapy for first-line H. pylori eradication.
This phase III trial investigated tegoprazan's efficacy and safety as part of the first-line triple therapy regimen for Helicobacter pylori (H. pylori) eradication.
In this randomized trial, patients diagnosed with H. pylori infection were assigned to receive either tegoprazan 50 mg (potassium-competitive acid blocker [P-CAB]) or lansoprazole 30 mg (proton pump inhibitor [PPI]), both combined with amoxicillin 1 g and clarithromycin 500 mg, twice daily for 7 days. The primary outcome was the elimination rate of H. pylori. Based on the cytochrome P450 (CYP) 2C19 genotype, the presence of underlying gastric conditions, and the minimum inhibitory concentrations (MIC) of clarithromycin and amoxicillin, subgroup assessments were conducted.
Among 350 enrolled patients, the eradication rates were strikingly close in the intention-to-treat and per-protocol analysis—demonstrating non-inferiority (Table 1).
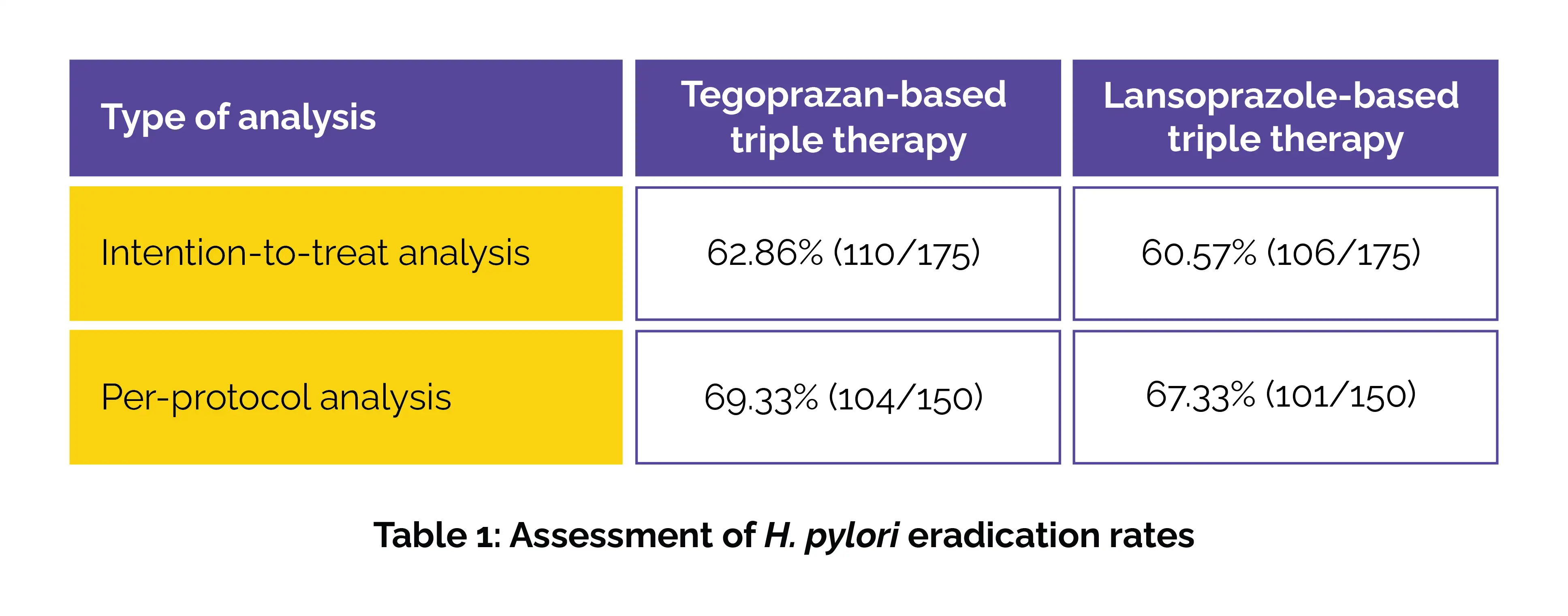
Subgroup analyses based on MIC values or CYP2C19 genotypes revealed no prominent differences in eradication outcomes. Both first-line triple therapies showed good tolerability, with no vital differences in safety profiles.
For first-line H. pylori management, tegoprazan-based triple therapy was as effective and safe as lansaprazole-based triple therapy. However, it did not address clarithromycin-resistant strains of H. pylori in Korea.
Gut liver
Triple Therapy-Based on Tegoprazan, a New Potassium-Competitive Acid Blocker, for First-Line Treatment of Helicobacter pylori Infection: A Randomized, Double-Blind, Phase III, Clinical Trial
Yoon Jin Choi et al.
Comments (0)