Categories
Change Password!
Reset Password!


The Phase 3 THRIVE-AA1 trial investigated the efficacy and safety of the oral janus kinase (JAK1/JAK2) inhibitor deuruxolitinib for the management of alopecia areata.
Deuruxolitinib (8 mg and 12 mg twice daily) significantly improves hair regrowth and patient satisfaction in adults with alopecia areata, with a favorable safety profile.
The Phase 3 THRIVE-AA1 trial investigated the efficacy and safety of the oral janus kinase (JAK1/JAK2) inhibitor deuruxolitinib for the management of alopecia areata.
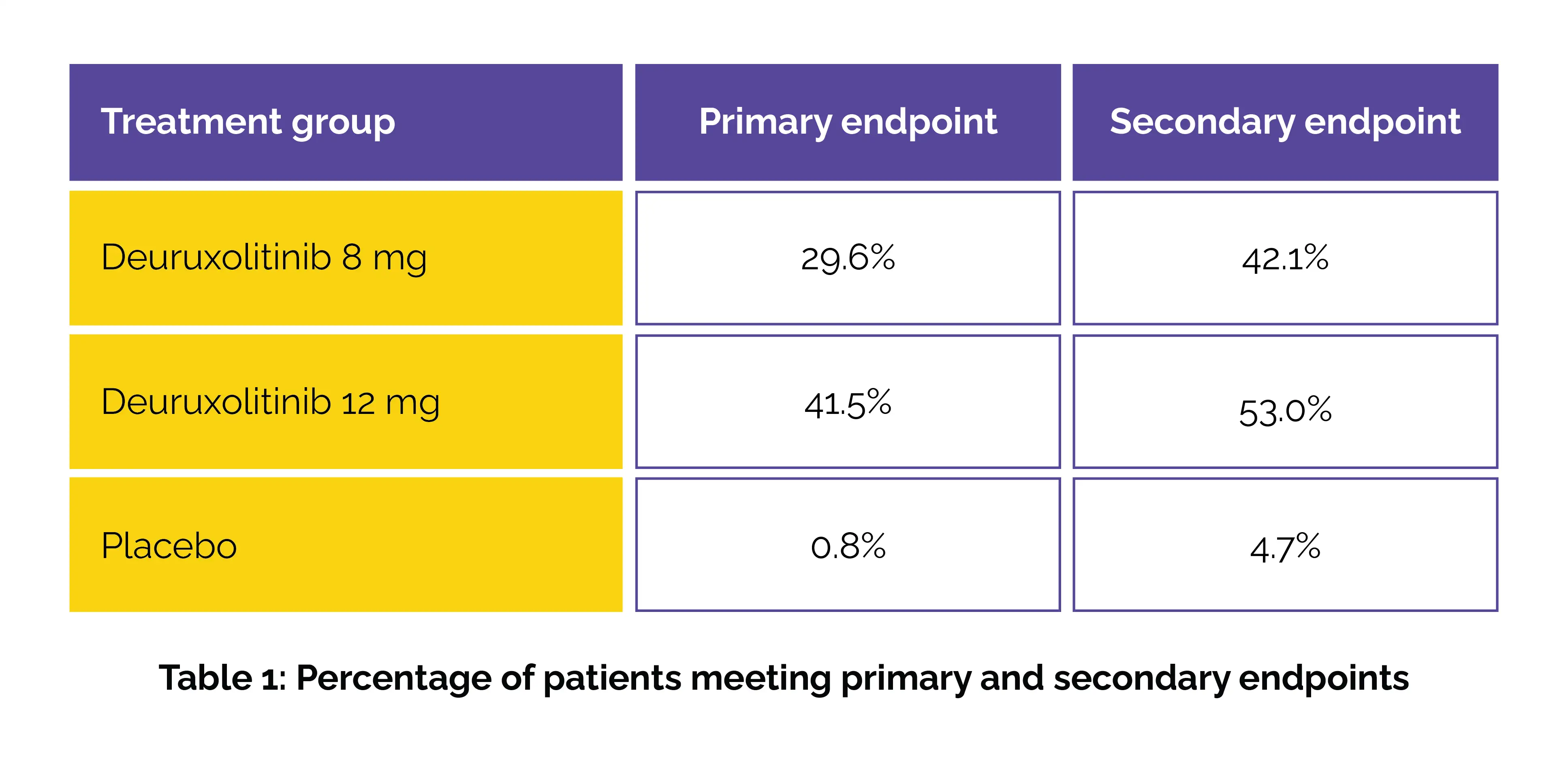
In this randomized controlled study, adults aged 18-65 with ≥50% hair loss were randomly allocated to get either deuruxolitinib 8 mg twice daily, 12 mg twice daily, or a placebo for 24 weeks. The percentage of participants attaining a Severity of Alopecia Tool (SALT) score ≤20 was the key outcome ascertained. The secondary outcome focused on patient satisfaction regarding their hair, as reported in self-assessments.
When compared to placebo, significantly more patients on deuruxolitinib reached the primary endpoint. Both deuruxolitinib doses also showed marked improvements across all secondary outcomes, including patient-reported hair satisfaction (Table 1).
Most treatment-related side effects were mild or moderate, in line with other JAK inhibitors.
Deuruxolitinib, at both 8 mg and 12 mg, proved effective in stimulating hair regrowth, with patient satisfaction closely aligning with the observed improvements in hair growth.
Journal of the American Academy of Dermatology
Efficacy and safety of deuruxolitinib, an oral selective Janus kinase inhibitor, in adults with alopecia areata: Results from the Phase 3 randomized, controlled trial (THRIVE-AA1)
Brett King et al.
Comments (0)