Categories
Change Password!
Reset Password!


Older adults suffering from atopic dermatitis (AD) often experience more diverse inflammatory patterns compared with younger patients.
Subcutaneous stapokibart effectively improves disease severity and itch and maintains a favorable safety profile in elderly people with moderate-to-severe atopic dermatitis.
Older adults suffering from atopic dermatitis (AD) often experience more diverse inflammatory patterns compared with younger patients. This study investigated the safety and efficiency of stapokibart, an anti–IL-4 receptor α (IL-4Rα) monoclonal antibody, across different age groups.
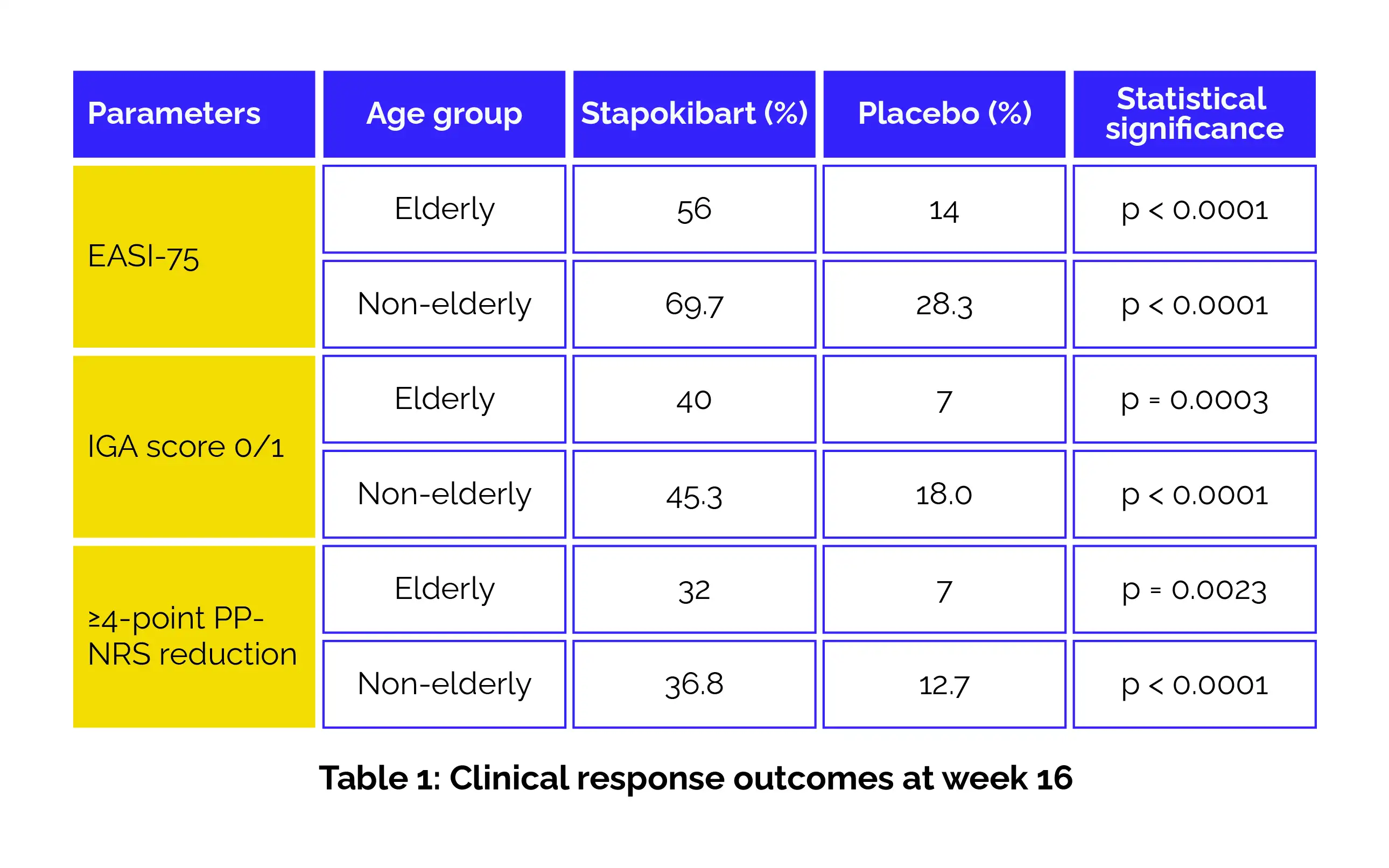
Adults with moderate-to-severe AD were randomized to get either stapokibart 300 mg subcutaneously every 2 weeks (following a 600 mg loading dose) or placebo for 16 weeks. After the initial period, all the volunteers received stapokibart 300 mg every 2 weeks for an additional 36 weeks. A post-hoc subgroup analysis classified volunteers as elderly (≥60 years; n=93) or non-elderly (18–59 years; n=407). The key endpoints included:
At week 16, stapokibart illustrated superior clinical effectiveness when compared with placebo across both age categories. Elderly and non-elderly patients receiving stapokibart exhibited higher response rates for EASI-75, IGA 0/1, and ≥4-point improvement in pruritus scores. Continued therapy during the maintenance phase led to sustained and progressive improvement in all measured outcomes (Table 1).
Treatment-emergent adverse events were comparable between stapokibart and placebo recipients during the 16-week controlled phase, and no new safety concerns emerged across age groups.
Stapokibart exhibited strong clinical efficacy and a favorable safety profile in older adults with AD, offering a promising targeted treatment option for this age group.
International Journal of Dermatology
Efficacy and Safety of Anti-IL-4Rα Stapokibart in Elderly Patients With Moderate-To-Severe Atopic Dermatitis
Yan Zhao et al.
Comments (0)