Categories
Change Password!
Reset Password!


A 14-day multicenter, randomized, double-blind, active-controlled, phase III clinical trial was conducted to assess the safety and effectiveness of the MP-AzeFlu (consists of Azelastine hydrochloride [AZE] and Fluticasone propionate [FLU]) nasal spray when compared to AZE and FLU nasal sprays in individuals with allergic rhinitis (AR).
MP-AzeFlu (one spray per nostril twice daily for 14 days) is effective in the symptomatic control of moderate-to-severe allergic rhinitis.
A 14-day multicenter, randomized, double-blind, active-controlled, phase III clinical trial was conducted to assess the safety and effectiveness of the MP-AzeFlu (consists of Azelastine hydrochloride [AZE] and Fluticasone propionate [FLU]) nasal spray when compared to AZE and FLU nasal sprays in individuals with allergic rhinitis (AR).
The study included adolescent and adult subjects with moderate-to-severe AR. The major efficacy outcome involved assessing the alteration from the baseline in the combined 12-hour reflective total nasal symptom score (rTNSS) comprising morning (AM) and afternoon (PM) symptoms.
The safety profile of the study medications was evaluated by recording, reporting, and analyzing baseline medical conditions, adverse events, vital signs, and conducting focused nasal assessments. Each treatment group encompassed 300 patients, resulting in a total sample size of 900 patients.
During the entire two-week treatment duration, the MP-AzeFlu group demonstrated a significant reduction in symptoms in rTNSS in comparison with both the AZE group (with a least square mean difference of -1.96) and the FLU group (with a least square mean difference of -0.98), as shown in Figure 1:
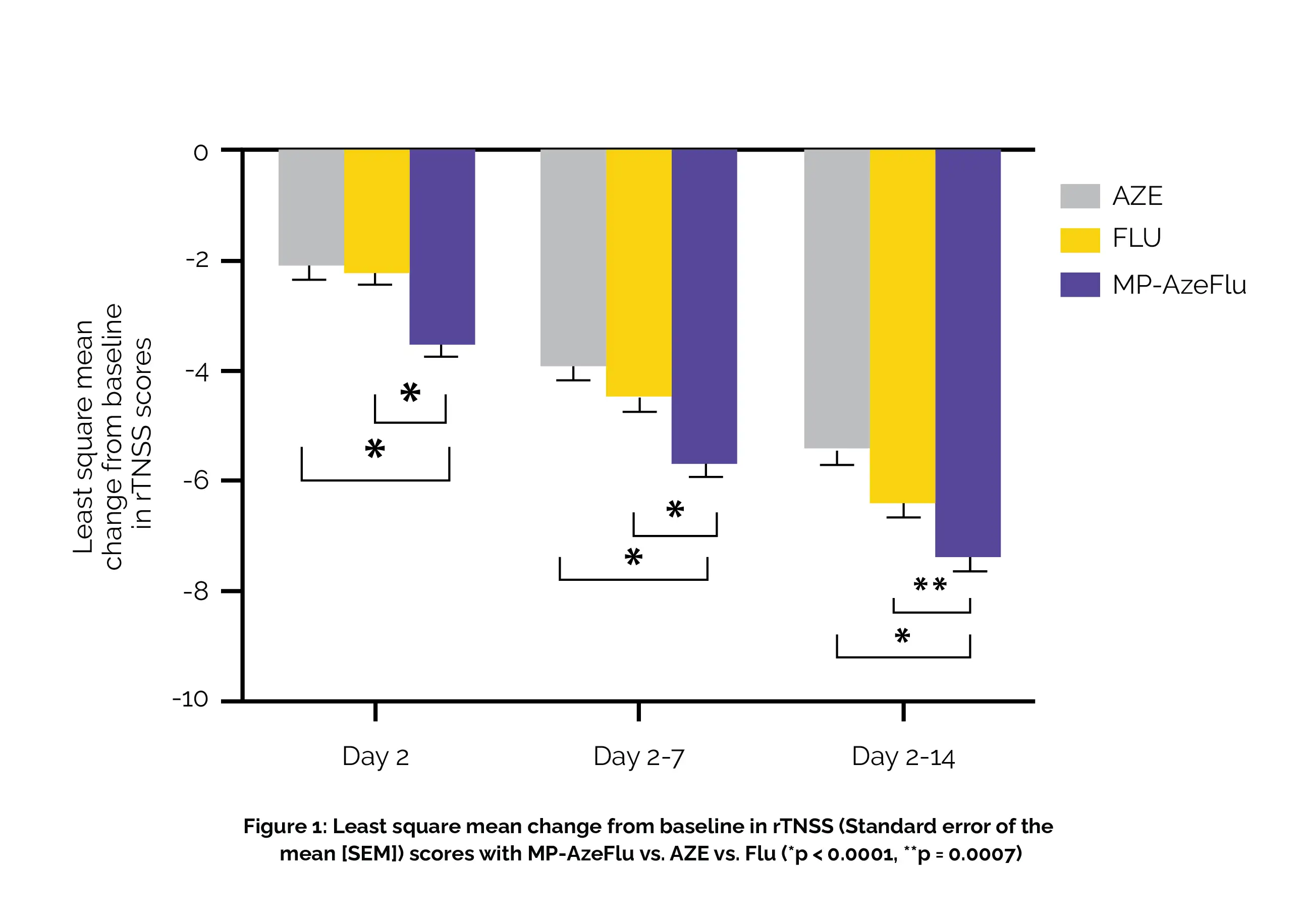
Additionally, the results of the adult Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) indicated a betterment in the quality of life in all treatment groups. The only noteworthy exception was dysgeusia (a bitter taste), which was reported by more subjects (13 individuals, or 4.3%) in the MP-AzeFlu group. In terms of the occurrence of all other treatment-emergent adverse events, the MP-AzeFlu group had similar or even lower rates compared to the other treatment groups.
The findings from this study substantiated the greater effectiveness and comparable safety characteristics of MP-AzeFlu nasal spray when compared to AZE or FLU in Chinese patients battling moderate-to-severe AR.
Pulmonary Therapy
A Clinical Study to Assess the Efficacy and Safety of MP-AzeFlu Nasal Spray in Comparison to Commercially Available Azelastine Hydrochloride and Fluticasone Propionate Nasal Sprays in Chinese Volunteers with Allergic Rhinitis
Bing Zhou et al.
Comments (0)