Categories
Change Password!
Reset Password!


Stapokibart markedly improves symptoms and itch relief in moderate-to-severe atopic dermatitis patients, regardless of whether they have type 2 inflammatory comorbidities.
A new post-hoc analysis from a phase 3 clinical trial has confirmed that stapokibart, a humanized monoclonal antibody targeting interleukin-4 receptor alpha, offers significant and sustained improvements in adults with atopic dermatitis (AD), including patients who also suffer from type 2 inflammatory comorbidities like asthma, allergic rhinitis, food allergies, chronic urticaria, or chronic obstructive pulmonary disease (COPD).
A total of 500 adults with moderate-to-severe AD were incorporated. During the initial 16-week double-blind period, participants received either:
After week 16, all patients transitioned to open-label stapokibart 300 mg every 2 weeks for an additional 36 weeks, totaling 52 weeks of treatment. Patients were grouped into two subpopulations:
Baseline demographics and disease severity scores were comparable between the groups. At week 16, stapokibart illustrated statistically significant superiority over placebo across all measured outcomes (Table 1).
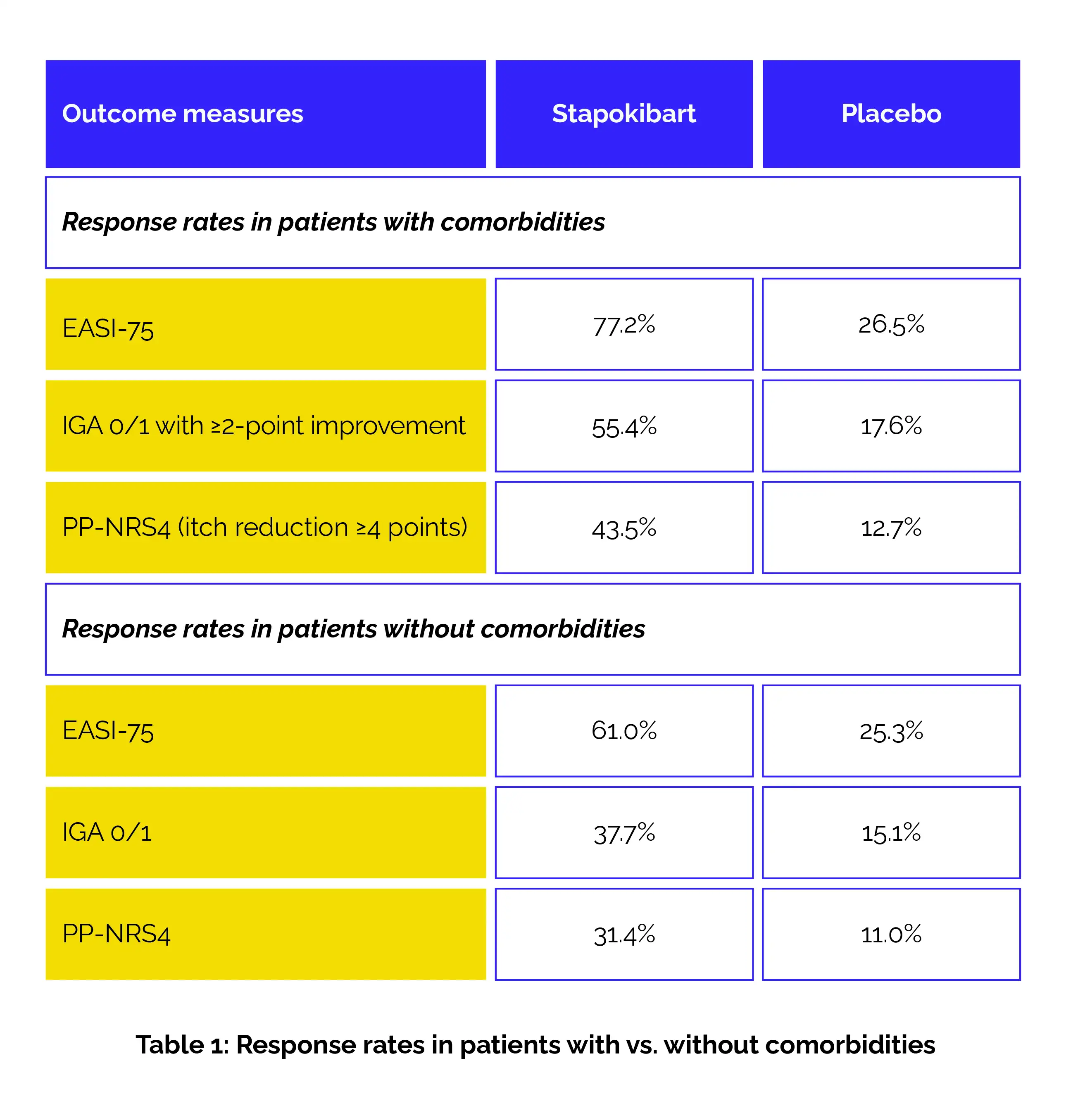
Importantly, the benefit extended through week 52, with continued improvement during long-term maintenance. Treatment-emergent adverse events (TEAEs) occurred at equivalent rates across both subgroups:
Notably, conjunctivitis, sometimes seen in biologic AD therapies, was reported in:
No new or unexpected safety signals emerged throughout the 52-week period. The findings demonstrate that stapokibart offers rapid, durable, and clinically meaningful improvements in eczema symptoms — whether or not patients have coexisting type 2 inflammatory diseases.
Clinical and Translational Allergy
Efficacy and Safety of Stapokibart in Adults With Moderate-to-Severe Atopic Dermatitis With and Without Type 2 Comorbidities: A Post Hoc Analysis of a Phase 3 Trial
Yan Zhao et al.
Comments (0)