Categories
Change Password!
Reset Password!


Vonoprazan-based triple therapy has illustrated high Helicobacter pylori (H. pylori) eradication success owing to its strong acid suppression as a potassium-competitive acid blocker (P-CAB).
Tegoprazan (100 mg triple therapy) effectively eradicates H. pylori at rates similar to vonoprazan (20 mg triple therapy) while tegoprazan (50 mg triple therapy) provides suboptimal results.
Vonoprazan-based triple therapy has illustrated high Helicobacter pylori (H. pylori) eradication success owing to its strong acid suppression as a potassium-competitive acid blocker (P-CAB). This study explored the efficiency and safety of tegoprazan-based triple therapy versus vonoprazan-based triple therapy in treatment-naive patients diagnosed with H. pylori disease.
This double-blind, multicenter pilot trial was conducted among adults newly diagnosed with H. pylori. Participants were randomly assigned in a 1:1:1 ratio to receive one of the following regimens twice daily for 10 days:
The primary endpoint was the H. pylori elimination rate assessed using the full analysis set (FAS) and the per-protocol (PP) set.
Of 102 recruited patients, 97 finished the randomized controlled trial. H. pylori eradication rates varied between groups, with higher doses of tegoprazan illustrating improved outcomes (Table 1).
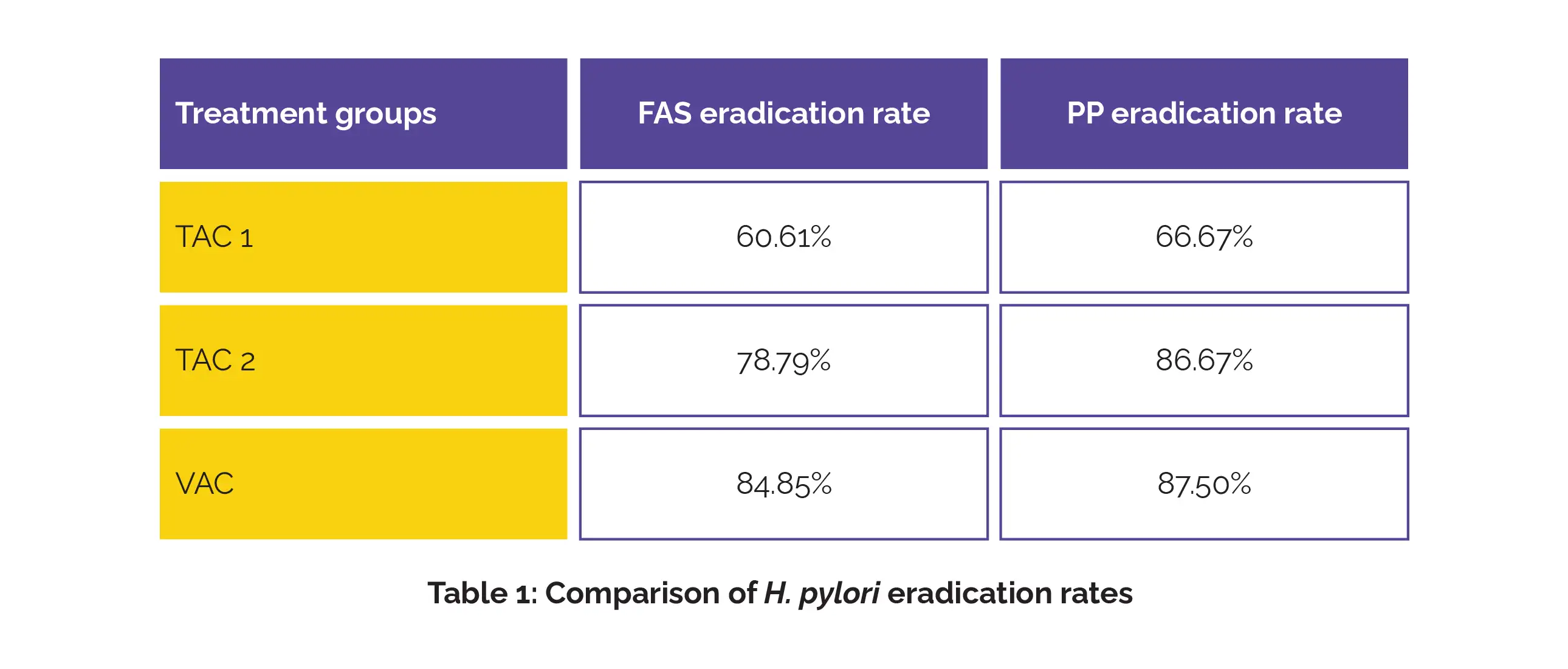
Comparative eradication rate differences showed:
All regimens were well-tolerated, with no clinically relevant difference in adverse events across groups.
A 10-day treatment with tegoprazan 100 mg-based triple therapy achieved similar H. pylori eradication rates to vonoprazan 20 mg-based triple therapy. But, tegoprazan 50 mg-based triple therapy appeared less effective.
Gut and Liver
Comparative Efficacy of Potassium-Competitive Acid Blocker-Based Triple Therapy with Tegoprazan versus Vonoprazan for Helicobacter pylori Eradication: A Randomized, Double-Blind, Active-Controlled Pilot Study
Jae Yong Park et al.
Comments (0)