Categories
Change Password!
Reset Password!


Fluticasone furoate/umeclidinium/vilanterol effectively and safely alleviates chronic cough and boosts lung function in asthma patients within 6 weeks.
A new trial suggests that triple therapy with fluticasone furoate, umeclidinium, and vilanterol (FF/UMEC/VI) successfully reduces persistent cough in asthma patients, improving both symptom control and lung function.
Persistent cough in asthma negatively impacts the quality of life, yet its optimal treatment remains unclear. Hence, a randomized, double-blind, placebo-controlled trial investigated FF/UMEC/VI dry powder inhaler for tackling adult asthma patients suffering from recurrent cough. In total, 60 volunteers received either FF/UMEC/VI (200/62.5/25 µg) or placebo for 6 weeks.
The main endpoint was alteration in cough symptom scores from baseline to week 6. Secondary outcomes encompassed the impact on cough-related disease burdens, assessed using the Asthma Control Questionnaire (ACQ-5), Leicester Cough Questionnaire (LCQ), and frequency of nighttime awakenings. Additionally, lung function and occurrence of adverse events were examined. As found, FF/UMEC/VI led to a greater reduction in cough symptom scores at week 6 (Figure 1).
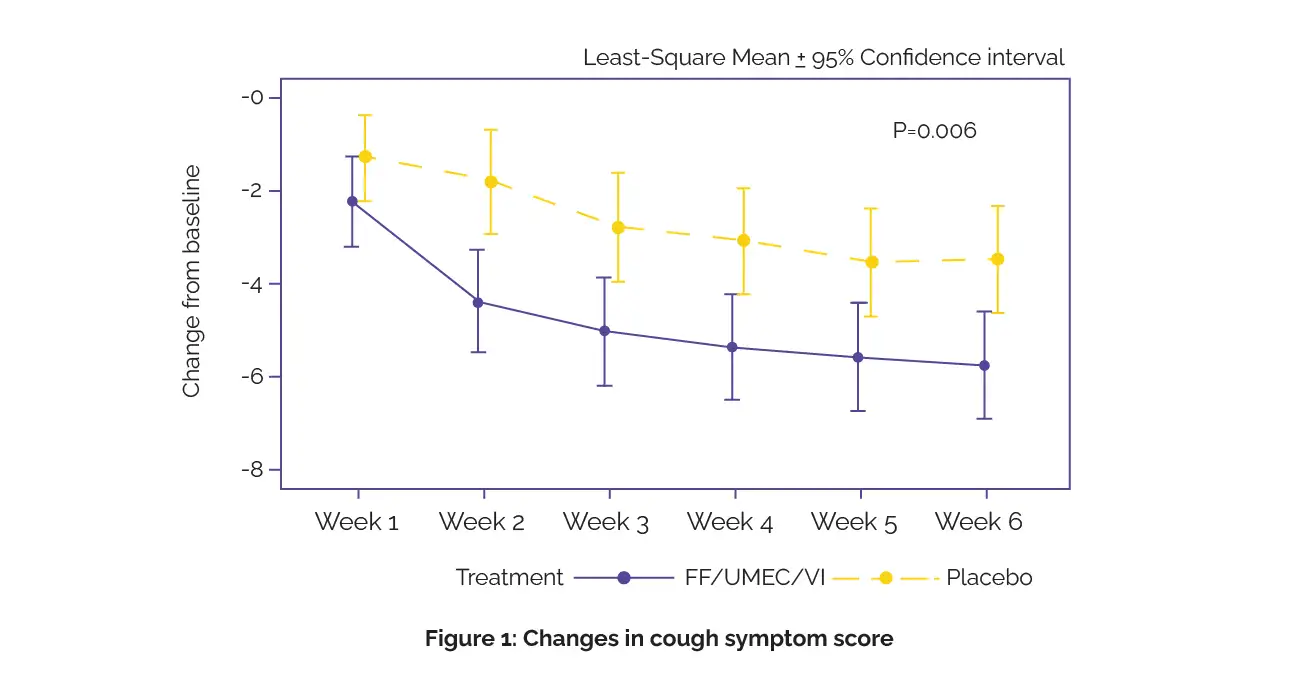
ACQ-5 improved more in the FF/UMEC/VI group than in the placebo group. Lung function markers, such as morning and evening forced expiratory volume (FEV1) and peak expiratory flow, improved with FF/UMEC/VI therapy, indicating broader respiratory benefits. Importantly, no significant adverse events were associated with triple therapy. These results suggest that FF/UMEC/VI provides early and effective relief for persistent cough in asthma while improving lung function without safety concerns.
The Journal of Asthma
The efficacy and safety of Fluticasone Furoate/Umeclidinium/vilanterol (FF/UMEC/VI) on cough symptoms in adult patients with asthma, a randomized double-blind, placebo-controlled, parallel group study: Chronic Cough in Asthma (COCOA) study
Etsuko Tagaya et al.
Comments (0)