Categories
Change Password!
Reset Password!


In children aged < 18 years old, the addition of Saccharomyces boulardii to standard triple therapy is associated with increased H. pylori eradication rates and a simultaneous decrease in both overall and gastrointestinal adverse events.
The findings of a systematic review and meta-analysis published in "BMC Infectious Diseases" presented compelling evidence that Saccharomyces boulardii (S. boulardii) supplementation, when incorporated into standard triple therapy (STT), not only enhances the eradication of Helicobacter pylori (H. pylori) in children but also significantly reduces the occurrence of adverse events. This can mark a revolutionary transformation in the approach to ameliorating H. pylori infections in pediatric patients.
The study sought to determine the effect of S. boulardii (non-pathogenic yeast) supplementation on H. pylori management in children aged < 18 years old. Researchers carried out electronic searches in Wanfang, PubMed, China National Knowledge Infrastructure, Embase, and the Cochrane Library database, covering the period up to September 2023. The pooled relative risk (RR) was calculated along with 95% confidence intervals (CI) via meta-analysis utilizing a random-effects model.
This research, encompassing 15 randomized controlled trials (RCTs) with a total of 2156 volunteers, revealed compelling evidence supporting the efficacy of S. boulardii when combined with STT (proton pump inhibitor [PPI] in combination with two antibiotics [Amoxicillin and Clarithromycin or Metronidazole]). As found, S. boulardii supplementation group exhibited a higher eradication rate compared to the STT alone group in both intention-to-treat analysis and per-protocol analysis, as shown in Table 1:
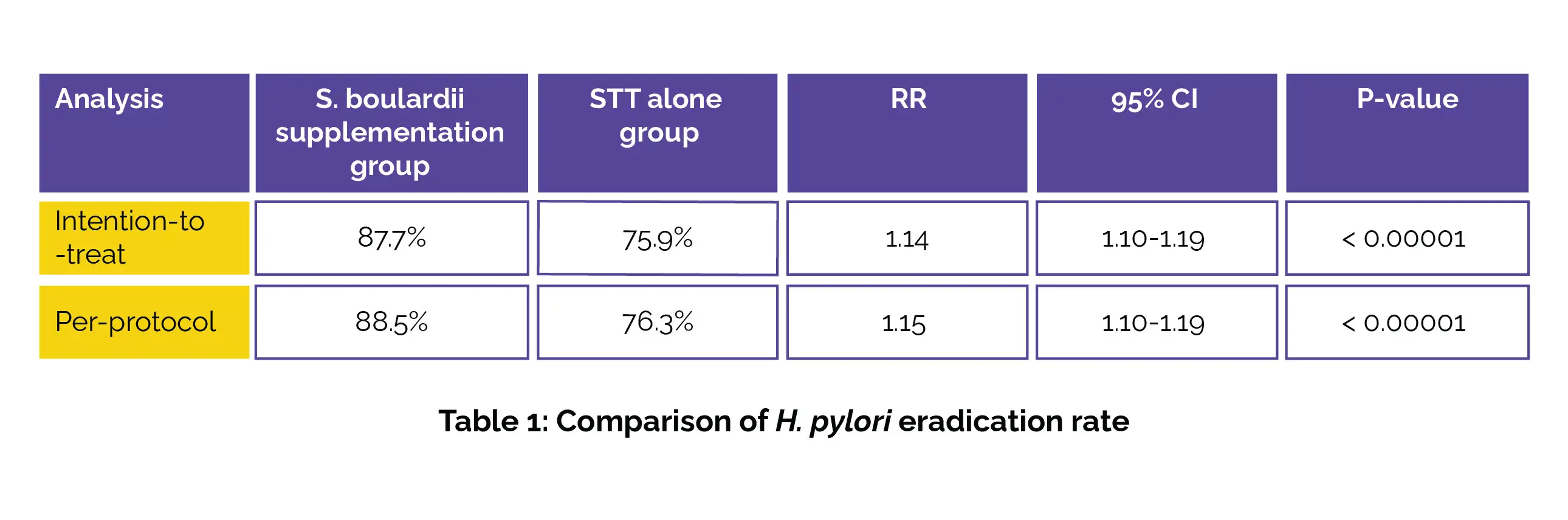
Moreover, the S. boulardii supplementation group demonstrated a noteworthy drop in the overall incidence of adverse events. Specifically, the incidence of total adverse events, diarrhea, and nausea was substantially lower in the S. boulardii supplementation group compared to the STT-alone group, as depicted in Table 2:
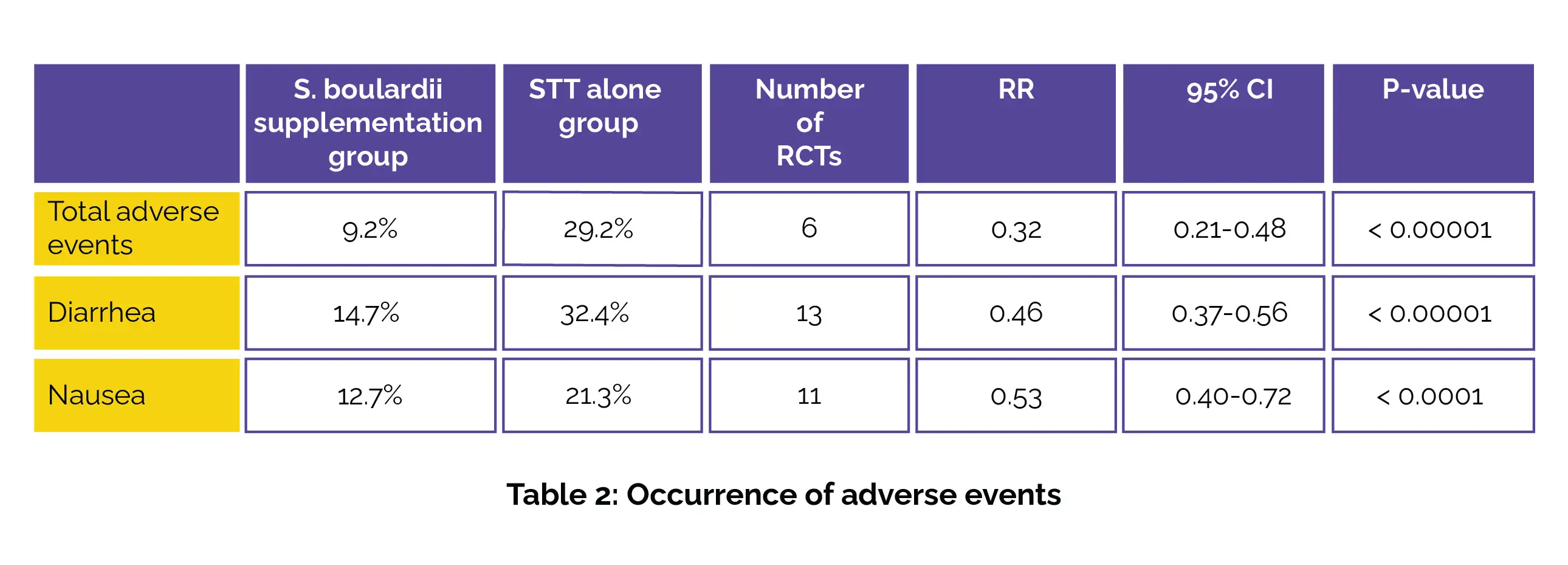
The findings also extended to various gastrointestinal adverse events, including stomatitis, vomiting, constipation, poor appetite, abdominal pain, epigastric discomfort, and abdominal distention. In each of these categories, the S. boulardii supplementation group exhibited lower incidence rates compared to the STT-alone group, providing a comprehensive picture of the potential benefits of S. boulardii use in pediatric H. pylori cases.
In a nutshell, children receiving S. boulardii (fungal probiotic) alongside STT exhibit improved H. pylori eradication, coupled with a reduction in total adverse events and gastrointestinal side effects.
BMC Infectious Diseases
The effect of Saccharomyces boulardii supplementation on Helicobacter pylori eradication in children: a systematic review and meta-analysis of Randomized controlled trials
Lian-Hua Liu et al.
Comments (0)