Categories
Change Password!
Reset Password!


Elobixibat maintains long-term safety and efficacy in chronic constipation, including elderly patients, with high patient satisfaction and sustained improvements in defecation.
A final analysis of postmarketing surveillance has confirmed the long-term safety, efficacy, and tolerability of elobixibat (bile acid transporter inhibitor) used for chronic constipation. While an interim report previously investigated the 4-week outcomes in approximately 1,000 patients, this study provided comprehensive 52-week data from a larger population of around 3,000 patients in Japan. The findings offered valuable insights, particularly regarding the drug’s effects in elderly patients, whose long-term safety and efficacy data had remained unclear.
The surveillance encompassed 2 observation periods: a 4-week treatment period and a 52-week treatment period. Researchers analyzed adverse drug reactions (ADRs) and efficacy outcomes, including defecation frequency, stool consistency (Bristol Stool Form Scale), and patient satisfaction. In the 4-week safety analysis, 3,638 volunteers (mean age 70.8 years) were incorporated. Notably, 73.7% of volunteers were aged 65 or older. Most patients (62.5%) received elobixibat as monotherapy, while the remaining patients used it alongside other laxatives.
ADRs were reported in 6.35% of patients, with gastrointestinal ailments being the most common. Diarrhea (3.35%) and abdominal pain (2.06%) were the primary complaints. The incidence of ADRs varied with age, occurring in 5.49% of patients aged 65 and older, 4.85% of those aged 75 and older, and 2.80% of those aged 85 and older, suggesting a trend of fewer ADRs in older patients.
Over the 52-week period, the safety profile remained consistent, with ADRs reported in 5.40% of the 1,315 patients analyzed. No novel safety concerns emerged, reinforcing elobixibat’s tolerability for long-term use. Efficacy assessments exhibited prominent improvements in defecation frequency and stool consistency from week 2 onward, regardless of patient age or administration timing (before breakfast, lunch, or dinner). Patient satisfaction also increased steadily, as shown in Table 1:
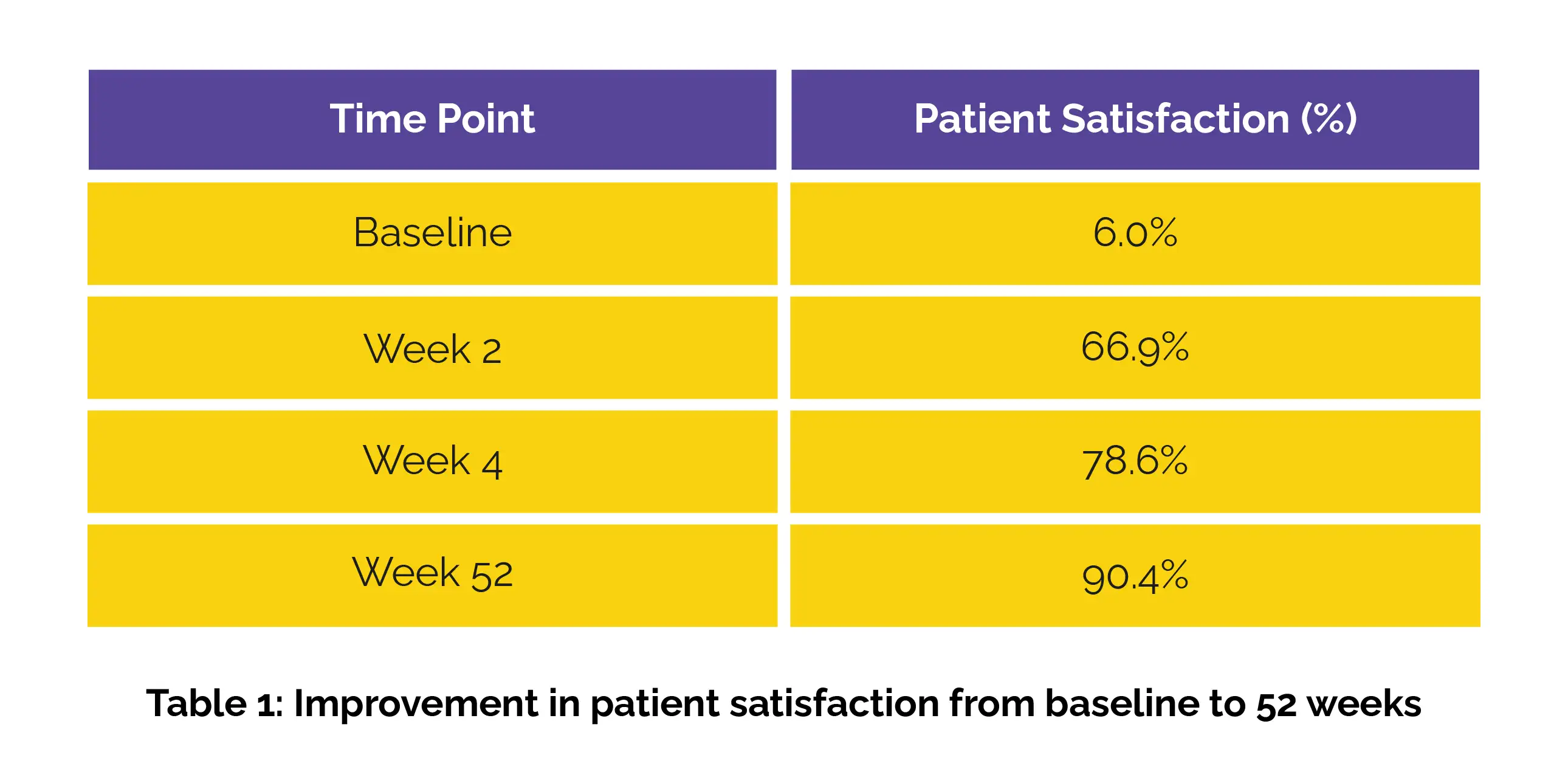
These findings reinforce elobixibat’s role as an effective, safe, and well-tolerated therapy to improve bowel function across different age groups.
SAGE Open Medicine
A multicenter, postmarketing surveillance of elobixibat in patients with chronic constipation in Japan: A final analysis report
Atsushi Nakajima et al.
Comments (0)