Categories
Change Password!
Reset Password!


Rifasutenizol-based triple therapy achieves over 90% H. pylori eradication with fewer adverse events than standard therapy.
A novel treatment strategy using rifasutenizol, a first-in-class compound with a synergistic dual mechanism of action, has shown promising results in eradicating Helicobacter pylori (H. pylori) infection. Findings from the phase 3 EVEREST-HP trial underscore its potential as an effective and safer alternative to standard regimens.
The trial, conducted across 40 centers, compared rifasutenizol-based triple therapy (RTT) with the conventional bismuth plus clarithromycin-based triple therapy (BCTT). A total of 700 treatment-naive patients (between the ages of 18 and 65), all confirmed positive for H. pylori through 13C-urea breath test and histological analysis, were randomized to receive:
Both regimens were given twice daily for about 14 days. The primary endpoint was infection elimination based on a follow-up 13C-urea breath test performed 4-6 weeks after treatment completion. Overall, 1,267 patients were screened, of whom 700 were randomized—353 assigned to RTT and 347 to BCTT. Cultures of H. pylori were successfully procured from 579 patients, revealing high resistance rates to clarithromycin (40.8%), metronidazole (68.2%), and levofloxacin (35.1%), while resistance to amoxicillin remained low at 8.1%.
Importantly, all isolates were susceptible to rifasutenizol. In the modified intention-to-treat (mITT) analysis, RTT attained a higher eradication rate when compared to the BCTT group. The difference of 4.2% met the non-inferiority threshold. Adverse events were also less frequent with RTT, as illustrated in Table 1:
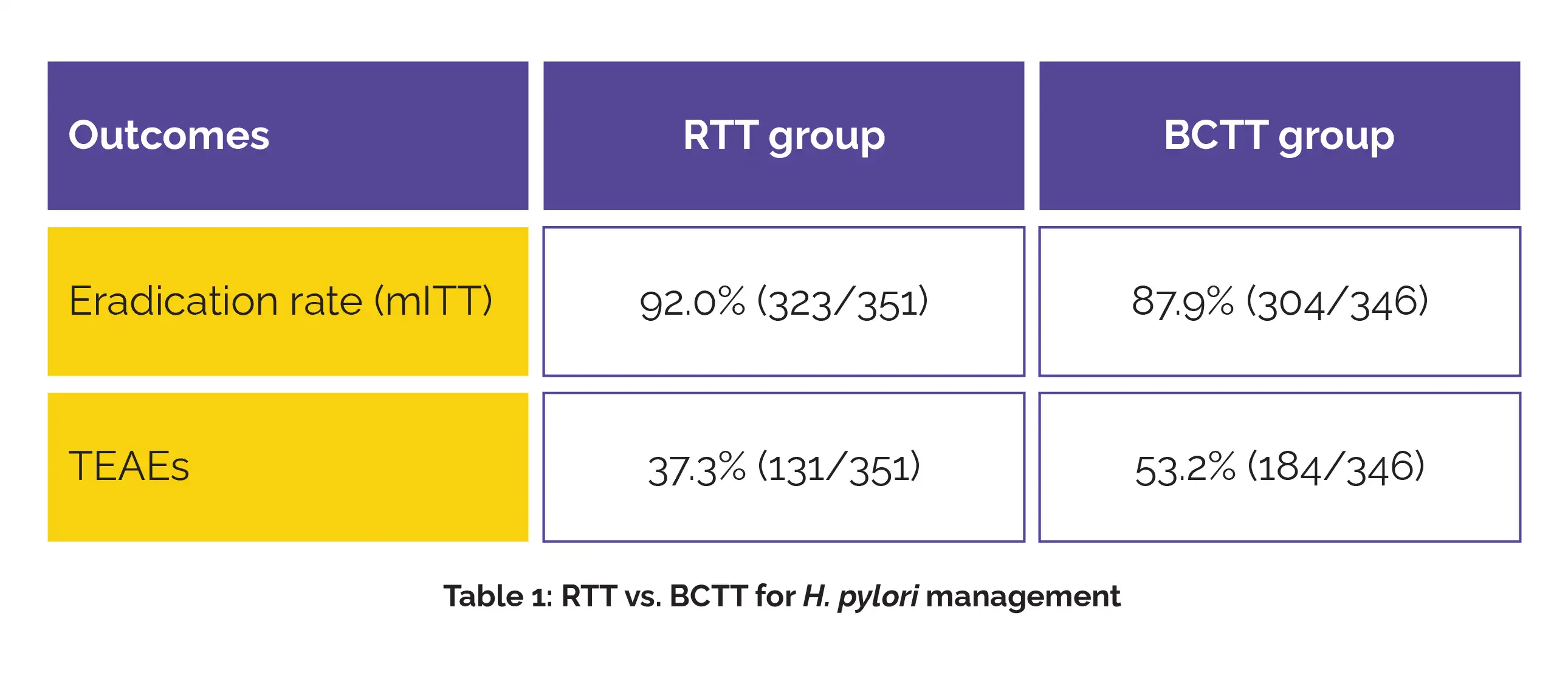
The most common side effects in the RTT arm included dizziness (6.0%), nausea (6.3%), and diarrhea (6.8%), while BCTT was more frequently linked with taste disturbances (36.1%), as well as nausea (6.1%) and diarrhea (5.5%). Most events were mild or moderate, and no serious adverse events associated with the study drugs were noted.
To sum up, rifasutenizol-based therapy is non-inferior to clarithromycin-based triple therapy and yields better tolerability, with H. pylori elimination rates exceeding 90% in treatment-naive patients. Thus, rifasutenizol may provide an important new option for first-line management of H. pylori, particularly in regions with high rates of antibiotic resistance.
The Lancet
Rifasutenizol, a Novel Antimicrobial Agent with a Dual Mechanism of Action, for the First-Line Treatment of Helicobacter Pylori Infection (EVEREST-HP): A Multicenter, Randomized, Double-Blind, Controlled, Non-Inferiority Phase 3 Trial
Zhiqiang Song et al.
Comments (0)