Categories
Change Password!
Reset Password!


Ecnoglutide, a novel GLP-1 receptor agonist, produces meaningful weight loss in adults with overweight or obesity while maintaining a favorable safety profile.
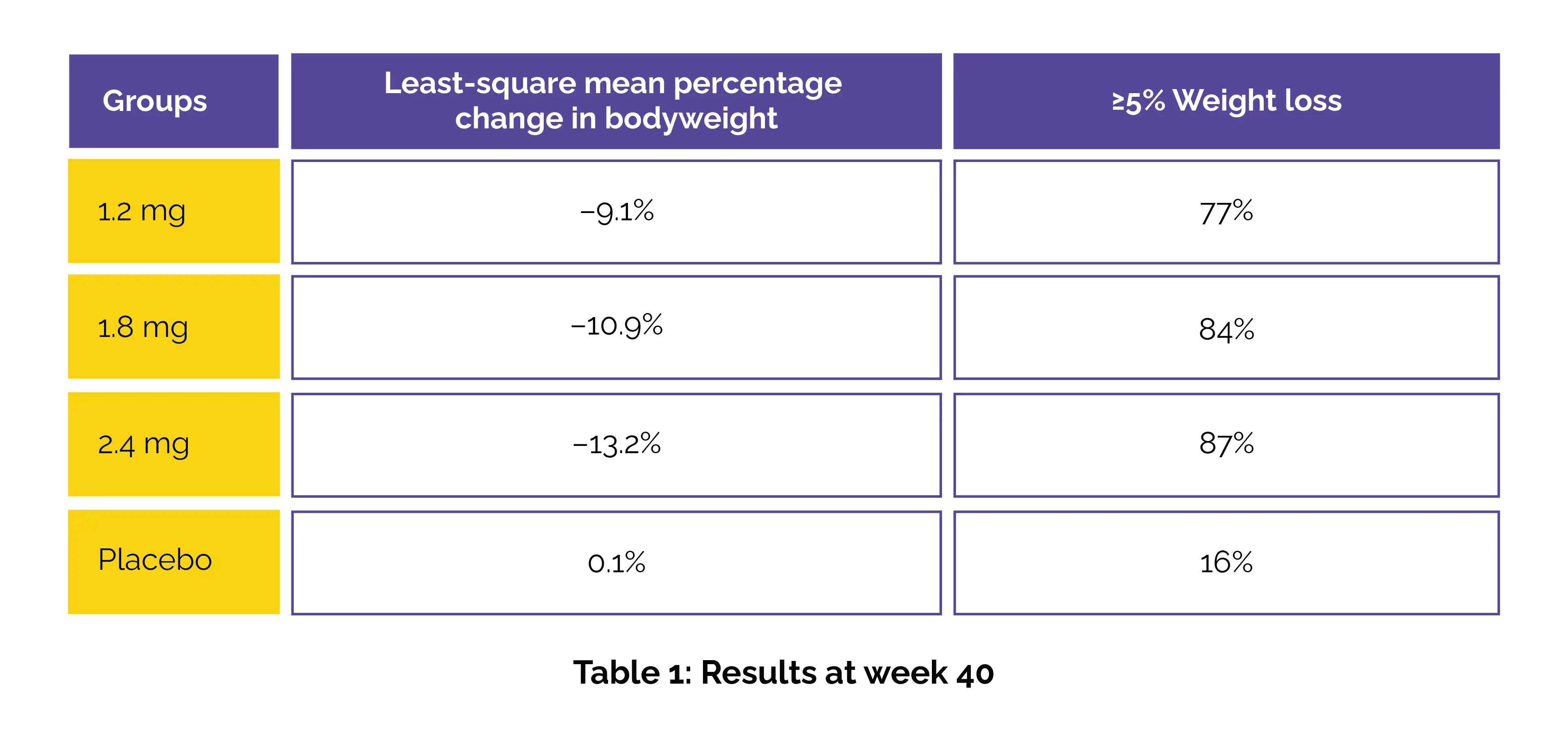
Adults living with obesity or overweight lost as much as 13.2% of their body weight after 40 weeks of treatment with the investigational drug ecnoglutide, according to results from a major phase 3 study conducted in China.
Ecnoglutide, a novel cyclic adenosine monophosphate (cAMP)-biased glucagon-like peptide-1 (GLP-1) receptor agonist, was tested in 664 adults (aged 18–75 years) with overweight or obesity. Eligible participants had at least one weight-related health condition, such as prediabetes, high blood pressure, high cholesterol, fatty liver, sleep apnoea, or joint pain. Importantly, none had type 1 or type 2 diabetes.
Participants received once-weekly injections of ecnoglutide (1.2 mg, 1.8 mg, or 2.4 mg) or placebo for 40 weeks. Co-primary endpoints were percentage bodyweight change and achievement of ≥5% weight loss. The results revealed superior reductions in weight with ecnoglutide across all doses compared with placebo (Table 1).
Adverse events were common but mostly mild-to-moderate gastrointestinal issues. Reported events occurred in:
Only 10 patients discontinued therapy due to side effects, underscoring an overall favorable safety profile. To sum up, subcutaneous ecnoglutide produces lasting weight loss with manageable side effects in adults with overweight or obesity without diabetes.
The Lancet Diabetes & Endocrinology
Efficacy and safety of a biased GLP-1 receptor agonist ecnoglutide in adults with overweight or obesity: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial
Linong Ji et al.
Comments (0)