Categories
Change Password!
Reset Password!


This study aimed to evaluate the correlation between Clopidogrel, Aspirin, and their combination with the progression of gastrointestinal harm in individuals (aged 18 to 80 years) without a high bleeding risk following percutaneous coronary intervention (PCI).
Following PCI, the use of Aspirin, Clopidogrel, or a combination of both is linked to an increased risk of progressive gastric and small-intestinal injury. This risk is particularly notable during dual antiplatelet therapy when compared to using either Aspirin or Clopidogrel alone.
This study aimed to evaluate the correlation between Clopidogrel, Aspirin, and their combination with the progression of gastrointestinal harm in individuals (aged 18 to 80 years) without a high bleeding risk following percutaneous coronary intervention (PCI).
Data for this study was obtained from a double-masked, placebo-controlled, multicenter randomized clinical trial (Optimal Antiplatelet Therapy for Prevention of Gastrointestinal Injury Evaluated by ANKON Magnetically Controlled Capsule Endoscopy; OPT-PEACE). People suffering from stable coronary artery disease or acute coronary syndromes without ST-segment elevation post-PCI and intended to undergo dual antiplatelet therapy (DAPT) for a minimum of six months, were enrolled.
Following the initial six-month DAPT period, participants without apparent bleeding or ischemic events underwent a second magnetically controlled capsule endoscopy (MCE) assessment. Among eligible subjects without gastrointestinal ulcers or bleeding (erosions were permitted) in the first two MCEs, constituting the intention-to-treat (ITT) population, random assignment (1:1:1) was carried out. For the subsequent six months, the volunteers were allocated to get:
A third MCE was conducted twelve months post-PCI. The main focus was to assess gastric injury advancement conducted at baseline, six months, and twelve months in the modified intention-to-treat (mITT) population through three MCEs, while the secondary endpoint was to evaluate the rate of small-intestinal harm advancement, marked as a quantitative rise in erosions or ulcers between the 2nd and 3rd MCEs conducted at six and twelve months, respectively.
In this secondary analysis, 394 patients were included in the mITT cohort, with a mean (standard deviation [SD]) age of 56.9 (8.7) years, predominantly male (296 [75.1%]). Notably, Aspirin users had a reduced rate of gastric injury advancement compared to DAPT users (risk ratio [RR], 0.70 [95% CI, 0.49-0.99]). Similar to gastric injury, Aspirin users demonstrated a lower rate of small-intestinal injury progression compared to DAPT users (RR, 0.71 [95% CI, 0.50-0.99]), as shown in Table 1:
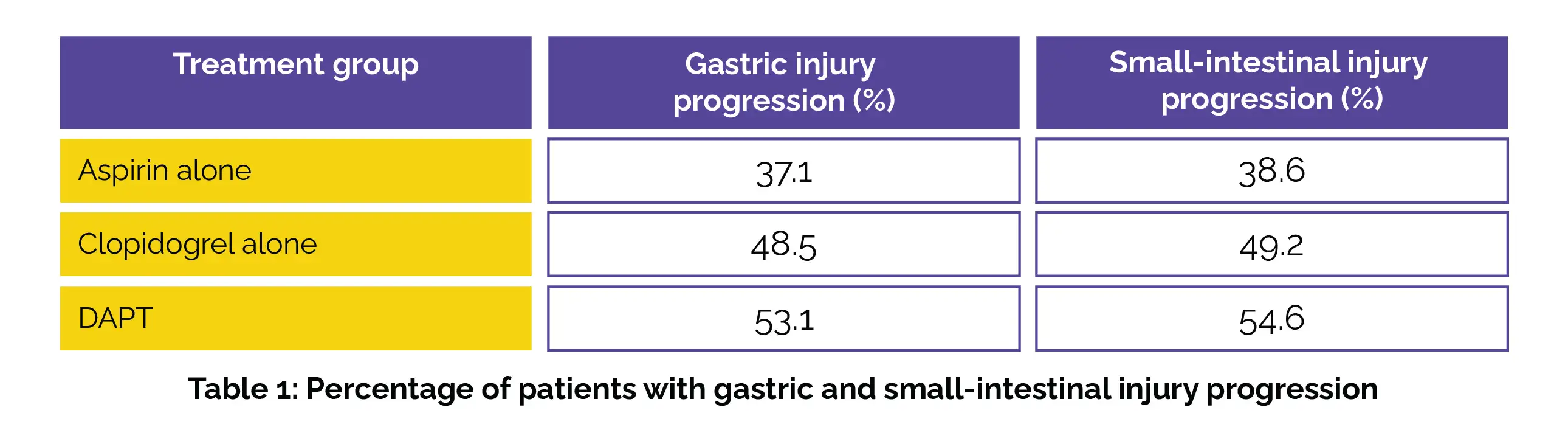
The continued use of Aspirin, Clopidogrel, or their combination from six to twelve months post-PCI was linked to a significant occurrence of advanced gastric and small-intestinal impairment in a considerable number of subjects, particularly with DAPT compared to monotherapy. Furthermore, Clopidogrel exhibited a likelihood of inducing gastrointestinal injury progression that was at least comparable to Aspirin.
JAMA Network Open
Progression of Gastrointestinal Injury During Antiplatelet Therapy After Percutaneous Coronary Intervention A Secondary Analysis of the OPT-PEACE Randomized Clinical Trial
Chen He et al.
Comments (0)