Categories
Change Password!
Reset Password!


Zuranolone functions as a positive allosteric modulator for gamma-aminobutyric acid (GABA) receptors, primarily developed to address postpartum depression (PPD), a common perinatal complication with deleterious infant and maternal outcomes. [1,2]
Zuranolone functions as a positive allosteric modulator for gamma-aminobutyric acid (GABA) receptors, primarily developed to address postpartum depression (PPD), a common perinatal complication with deleterious infant and maternal outcomes. [1,2]
It received FDA authorization for use on 4 August 2023, making it the exclusive approved treatment option for women dealing with PPD. [3] Zuranolone is a new compound that is responsible for the modulation of the activity of both synaptic and extrasynaptic GABAA receptors, which sets it apart from benzodiazepines, which specifically only target synaptic GABAA receptors. [4]
It was developed to exhibit the pharmacological characteristics of a neuroactive steroid, while also having the pharmacokinetic properties of a once-daily oral dosing formulation in mind.
Pharmacological Class: Neuroactive steroid [3]
Zuranolone is indicated for the management of PPD in adults. [3]
The precise way in which Zuranolone works to relieve PPD is not fully comprehended, but it is believed to be linked to its ability to positively influence GABAA receptors through allosteric modulation. In contrast to benzodiazepines, which are another category of GABAA positive modulators, Zuranolone doesn't target the α/γ subunit interface.
Instead, it binds to the α/β subunit interface, which is common to all GABAA receptors. Consequently, Zuranolone has the capacity to interact with both synaptic GABAA receptors, comprising 2α2βγ subunits, and extrasynaptic GABAA receptors, made up of 2α2βδ subunits. [3]
Absorption
After taking Zuranolone orally, the highest concentrations in the bloodstream are reached at around 5 to 6 hours (Tmax). When Zuranolone was consumed alongside a moderate-fat meal (equivalent to 700 calories with 30% fat content), the exposure to Zuranolone, as measured by Cmax and AUC, demonstrated a dose-dependent escalation. This increase was observed when transitioning from 30 mg to 60 mg, which is 1.2 times the recommended Zuranolone dose. Administering Zuranolone once a day led to an accumulation in systemic exposure by approximately 1.5 times, and a steady state in terms of drug levels was attained in 3-5 days.
In healthy individuals who received 30 mg of Zuranolone, the Cmax increased by approximately 3.5 times, and the AUClast elevated by about 1.8 times when it was taken with a low-fat meal (consisting of 400 to 500 calories with 25% fat) compared to taking it on an empty stomach. With a high-fat meal (containing 800 to 1,000 calories with 50% fat), the Cmax heightened by roughly 4.3 times, and the AUClast escalated by about 2 times compared to fasting conditions. The time it took to reach Tmax was not influenced by whether the medication was taken with food or on an empty stomach.
Volume of distribution
After oral administration, Zuranolone's volume of distribution exceeds 500 litres.
Protein binding
The mean blood-to-plasma concentration ratio falls within the range of 0.54 to 0.58. Zuranolone's binding to plasma proteins exceeds 99.5%.
Metabolism
Zuranolone undergoes significant metabolism, primarily by the CYP3A4 enzyme. However, there were no human metabolites in circulation that exceeded 10% of the total drug-related substances, and none of these metabolites are believed to play a role in Zuranolone's therapeutic effects.
Route of elimination
After administering radiolabeled Zuranolone orally, 45% of the administered dose was found in urine as metabolites, with very little unchanged Zuranolone, and 41% was detected in feces as metabolites, with less than 2% existing as unaltered Zuranolone.
Half-life
In adults, Zuranolone has an approximate terminal half-life ranging from 19.7 to 24.6 hours.
Clearance
Zuranolone's mean apparent clearance is 33 litres per hour. [3]
None
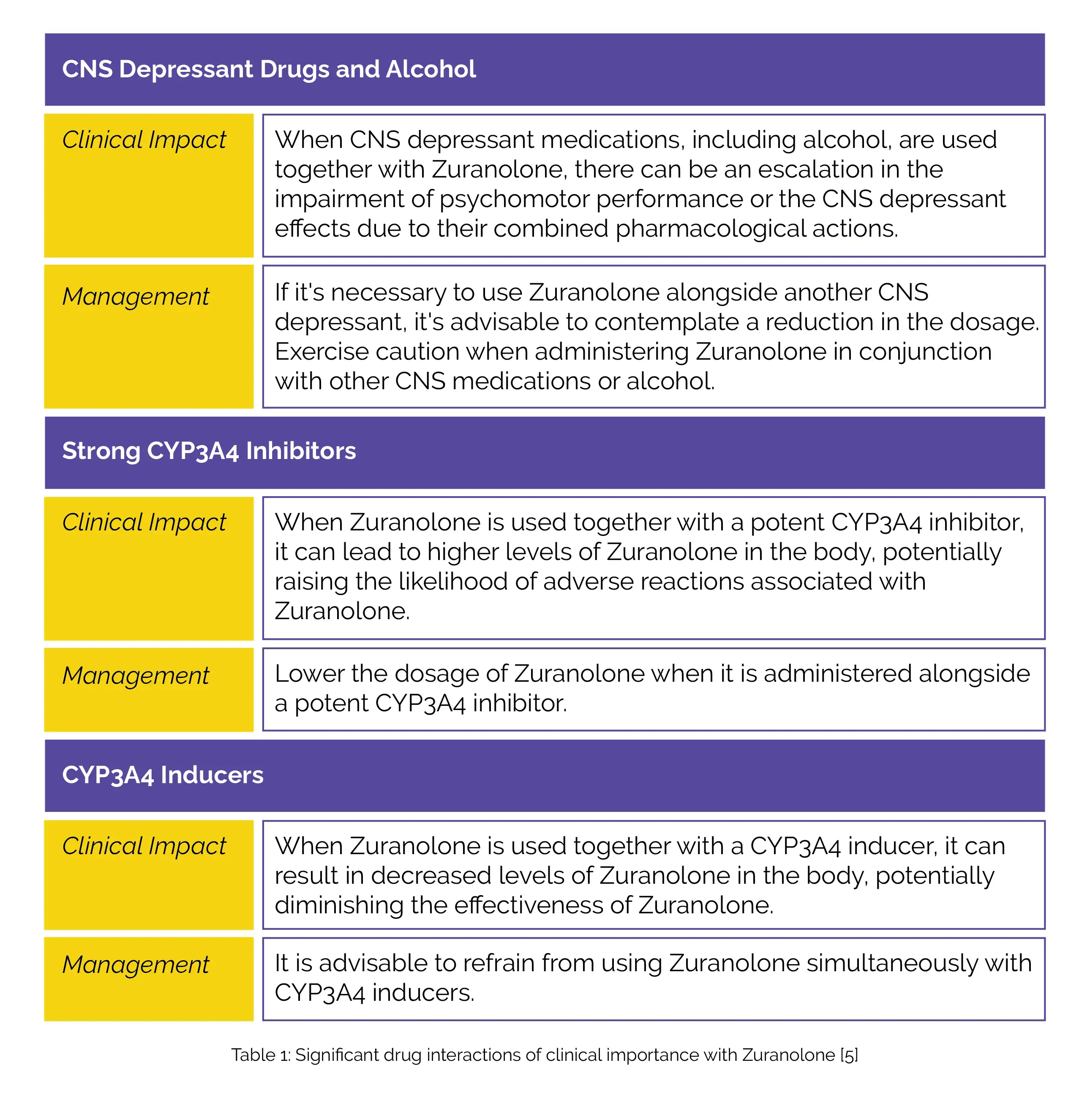
Table 1 illustrates clinically important drug interactions with Zuranolone.
The most frequent side effects associated with Zuranolone comprise:
In phase 3 randomized clinical trial carried out by Deligiannidis KM et al, Zuranolone showed promising results in relieving the primary symptoms of depression in women battling PPD. It was also reported to be well-tolerated, indicating its potential as a viable PPD treatment option. The main goal of the study was to assess how effective and safe Zuranolone is in treating PPD. The 151 participants in the study were women aged 18 to 45 who had given birth within the past six months and were currently experiencing PPD symptoms.
They were randomly assigned to receive either Zuranolone (30 mg) or a placebo orally every evening for a duration of 2 weeks. The major endpoint assessed was the change in Hamilton Rating Scale for Depression (HAMD-17) scores on day 15. Zuranolone exhibited remarkable improvements in HAMD-17 scores compared to placebo on day 15 (−17.8 vs. −13.6; a difference of −4.2). These improvements in favor of Zuranolone were consistently observed from day 3 (a difference of −2.7) through day 45 (a difference of −4.1).
Additionally, persistent differences in favor of Zuranolone were witnessed on day 15 in terms of HAMD-17 response (odds ratio, 2.63), HAMD-17 score remission (odds ratio, 2.53), alteration from baseline for the Montgomery-Åsberg Depression Rating Scale score (a difference of −4.6), and Hamilton Rating Scale for Anxiety score (a difference of −3.9). Majority of treatment-emergent adverse events were found to be mild or moderate. [1]
Another double-blind phase 3 trial assessed the effectiveness and safety of Zuranolone, as a 14-day oral treatment for severe PPD. Out of the 196 patients who were randomly assigned to either the Zuranolone group or the placebo group, those who received Zuranolone demonstrated notable symptomatic relief in depression when compared to the placebo group.
This betterment was evident not only on day 15 (least squares mean [LSM] alteration from baseline in HAM-D score, -15.6 vs. -11.6; LSM difference, -4.0) but also on days 45, 28, and 3. Zuranolone was generally well-tolerated, with commonly reported side effects including sedation, dizziness, and drowsiness. The study suggested that Zuranolone has the potential to be a rapid-acting oral treatment for PPD. [2]
In another phase 3 randomized controlled trial conducted by Deligiannidis KM et al, Zuranolone was evaluated for its impact on women aged 18-45 with severe PPD, including concurrent anxiety and/or insomnia symptoms. Participants were randomly assigned to receive either Zuranolone (30 mg daily for 14 days) or a placebo. The rates of achieving remission from both depressive and anxiety symptoms were higher when using Zuranolone compared to a placebo on days 3, 15, and 45. Additionally, the rate of sustained concurrent remission (meaning remission on both days 15 and 45) was also greater with Zuranolone use.
Anxiety symptoms, as examined by the Hamilton Depression Rating Scale (HDRS) -17 anxiety/somatization subscale and Edinburgh Postnatal Depression Scale anxiety subscale, showed improvement with Zuranolone use compared to placebo from day 3 through day 45. There were also potential benefits observed in alleviating insomnia symptoms and improving patients' perceived functional health. On day 15, the number needed to treat for achieving both HDRS-17 response and remission was 5.
In conclusion, Zuranolone demonstrated concurrent improvements in both depressive and anxiety symptoms, as well as potential benefits in addressing insomnia symptoms and enhancing the functional health perceived by adults with PPD. [7]
In the SKYLARK study, which investigated 50 mg Zuranolone as a potential treatment for PPD, positive results were obtained. The primary goal of the study was to measure the change in depression severity from baseline after 15 days of treatment using the HAMD-17 scale. The study involved patients aged 18-45 with severe PPD, and they were randomly assigned to receive either Zuranolone or a placebo for 14 days.
Results showed that patients who received Zuranolone experienced remarkable improvements in all HAMD-17 subscales compared to those who received the placebo at day 15. These improvements were noticeable as early as day 3 of treatment. In summary, the SKYLARK trial found that Zuranolone exhibited promising results in rapidly improving depressive and anxiety symptoms in PPD patients, suggesting its potential as a rapid-acting oral treatment option for this condition. [8]
1. Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, Doherty J, Jonas J, Li S et al. Effect of Zuranolone vs Placebo in Postpartum Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2021 Sep 1;78(9):951-959.
2. Deligiannidis KM, Meltzer-Brody S, Maximos B, Peeper EQ, Freeman M, Lasser R et al. Zuranolone for the Treatment of Postpartum Depression. American Journal of Psychiatry. 2023 Sep 1;180(9):668-675.
3. Zuranolone. Drug Bank. Accession Number DB15490. Available online from: https://go.drugbank.com/drugs/DB15490 [Last accessed on: 3 October 2023]
4. Patterson R, Balan I, Morrow AL, Meltzer-Brody S. Novel neurosteroid therapeutics for post-partum depression: perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacology. 2023 Sep 15:1-6.
5. Zuranolone. FDA LABEL. Available online from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217369s000lbl.pdf
[Last accessed on: 3 October 2023]
6. Mahase E. US approves daily pill for postpartum depression. BMJ. 2023 Aug 8;382:1822.
7. Deligiannidis KM, Citrome L, Huang MY, Acaster S, Fridman M, Bonthapally V et al. Effect of Zuranolone on Concurrent Anxiety and Insomnia Symptoms in Women With Postpartum Depression. The Journal of Clinical Psychiatry. 2023 Jan 30;84(1):22m14475.
8. Deligiannidis K, Bullock A, Kotecha M, Li S, Maximos B, Vera T. Improvement in HAMD-17 Subscale Scores With 14-Day Treatment Course of Zuranolone in Postpartum Depression: Results From the SKYLARK Study [ID: 1372313]. Obstetrics & Gynecology. 2023 May 1;141(5):64S-5S.
Comments (1)