Categories
Change Password!
Reset Password!


FDA approves rilzabrutinib tablets as a new oral treatment option for adults with persistent or chronic ITP who show insufficient response to standard therapies—marking a significant advancement in targeted ITP management.
Immune thrombocytopenia (ITP) is a chronic autoimmune ailment marked by unusually low platelet counts that can trigger bleeding and bruising. Despite available treatments like corticosteroids, immunoglobulins, and anti-D therapy, many adults fail to maintain adequate platelet levels. On 2 Sep 2025, the U.S. Food and Drug Administration (FDA) approved rilzabrutinib (a novel oral Bruton’s tyrosine kinase inhibitor) designed to provide a durable and clinically meaningful platelet response in adults with persistent or chronic ITP. The approval of rilzabrutinib aims to expand treatment options for those unresponsive to conventional therapy.
The safety and efficacy of rilzabrutinib were evaluated in a 24-week, double-blind, parallel-group clinical study assessing its ability to achieve a durable platelet response—defined as a sustained platelet increase from baseline during most of the final 12 weeks of treatment. A total of 202 adult patients with persistent or chronic ITP were randomly assigned to get either rilzabrutinib or a placebo. A higher proportion of patients in the rilzabrutinib group achieved a durable platelet response when compared to the placebo group (Table 1).
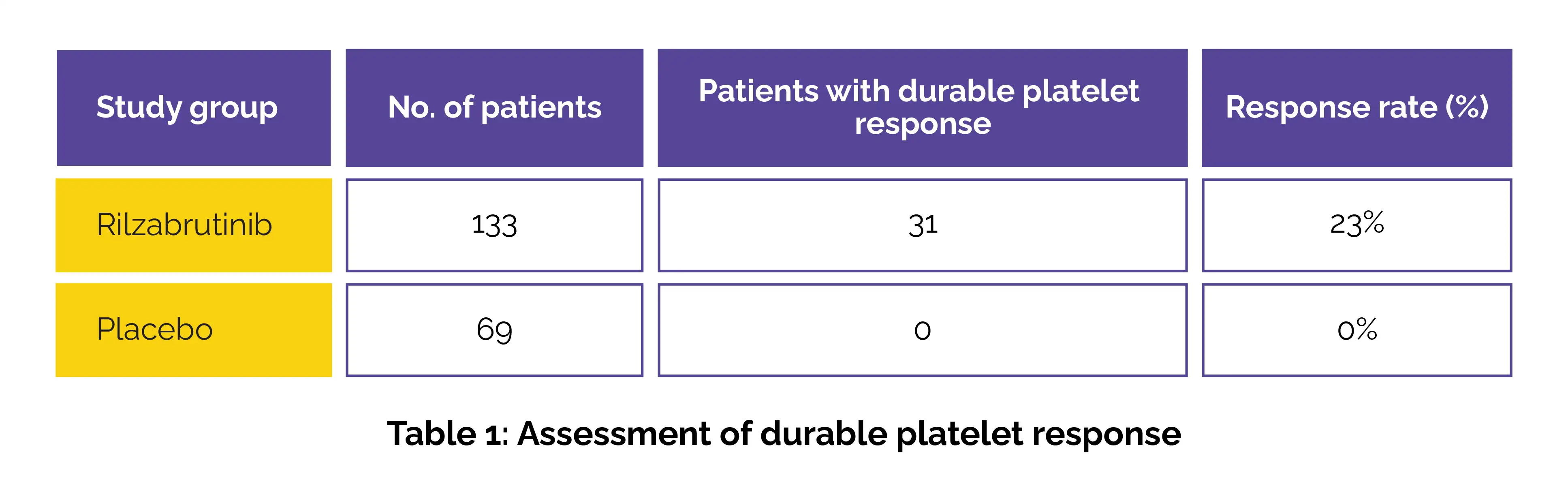
Moreover, an increased likelihood of serious infections, including bacterial, viral, and fungal types, was identified as a potential concern with rilzabrutinib. The most common adverse effects included abdominal pain, diarrhea, nausea, headache, and COVID-19. Patients experiencing gastrointestinal discomfort were advised to take the tablets with food for improved tolerance. The therapy demonstrated clinically meaningful platelet improvement, paving the way for a new standard in the management of this challenging autoimmune condition.
U.S. Food and Drug Administration
FDA Approves Rilzabrutinib to Treat Adults with Persistent or Chronic Immune Thrombocytopenia
Comments (0)