Categories
Change Password!
Reset Password!


This trial investigated the effectiveness and safety of metformin + myo-inositol versus metformin alone for the management of polycystic ovary syndrome (PCOS) symptoms.
Combining metformin with myo-inositol offers superior benefits over metformin alone in addressing insulin resistance and menstrual disturbances in PCOS.
This trial investigated the effectiveness and safety of metformin + myo-inositol versus metformin alone for the management of polycystic ovary syndrome (PCOS) symptoms.
Overall, 196 PCOS-affected adult females (18-40 years) were enrolled. Volunteers were randomly assigned to get either metformin hydrochloride (500 mg) + myo-inositol (600 mg) combination or metformin alone (500 mg) two times daily for about 24 weeks. In this phase III, randomized clinical trial, the key outcomes were:
At week 24, a higher proportion of patients in the metformin + myo-inositol group experienced improvements in HOMA-IR when compared to the metformin group. Additionally, fewer patients in the combination group experienced heavy menstrual bleeding (>80 mL), as depicted in Table 1:
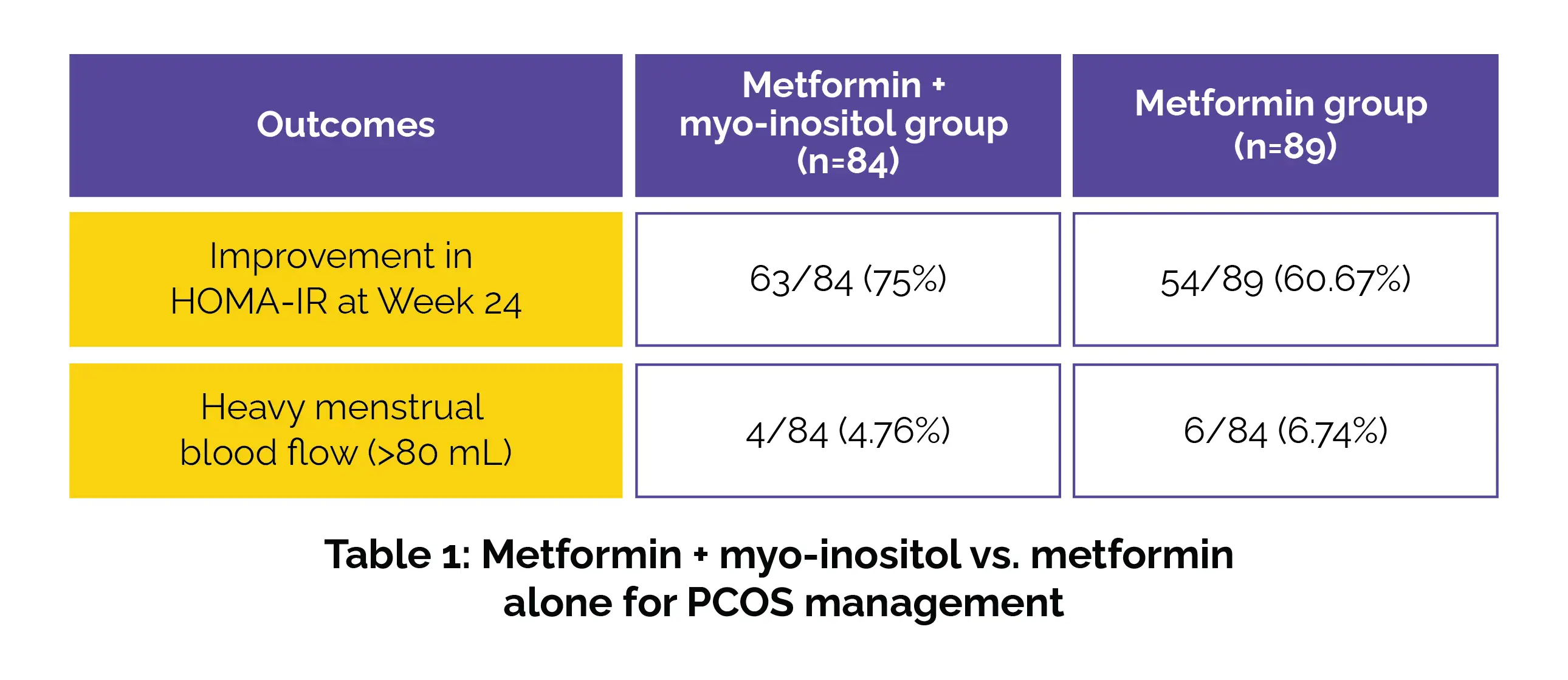
Menstrual cycle normalization and reduction in infrequent menstruation were also quite greater in the combination group. Adverse reactions were mild and comparable between the study groups.
The fixed-dose combination of metformin and myo-inositol outperformed metformin alone in improving insulin resistance and menstrual irregularities (especially the heaviness and frequency of menstruation) in PCOS sufferers, offering a promising strategy for better management of the condition.
Cureus
A Phase III, Double-Blind, Randomized, Multicenter, Clinical Trial to Evaluate the Efficacy and Safety of a Fixed-Dose Combination of Metformin Hydrochloride and Myo-Inositol Compared to Metformin in Patients With Polycystic Ovary Syndrome
Alka Kriplani et al.
Comments (0)