Categories
Change Password!
Reset Password!


Atopic dermatitis (AD) presents differently across skin tones, yet individuals with skin of color remain underrepresented in clinical research.
Once daily use of 1% tapinarof cream shows consistent efficacy and good tolerability across diverse atopic dermatitis populations, irrespective of race or Fitzpatrick skin type.
Atopic dermatitis (AD) presents differently across skin tones, yet individuals with skin of color remain underrepresented in clinical research. In the phase 3 ADORING 1 and 2 trials—8-week, randomized studies—tapinarof cream 1% once daily (QD) showed markedly greater efficacy than vehicle in adults and kids as young as 2 years with AD. The present analyses assess the efficacy of tapinarof cream 1% QD based on race and Fitzpatrick skin type.
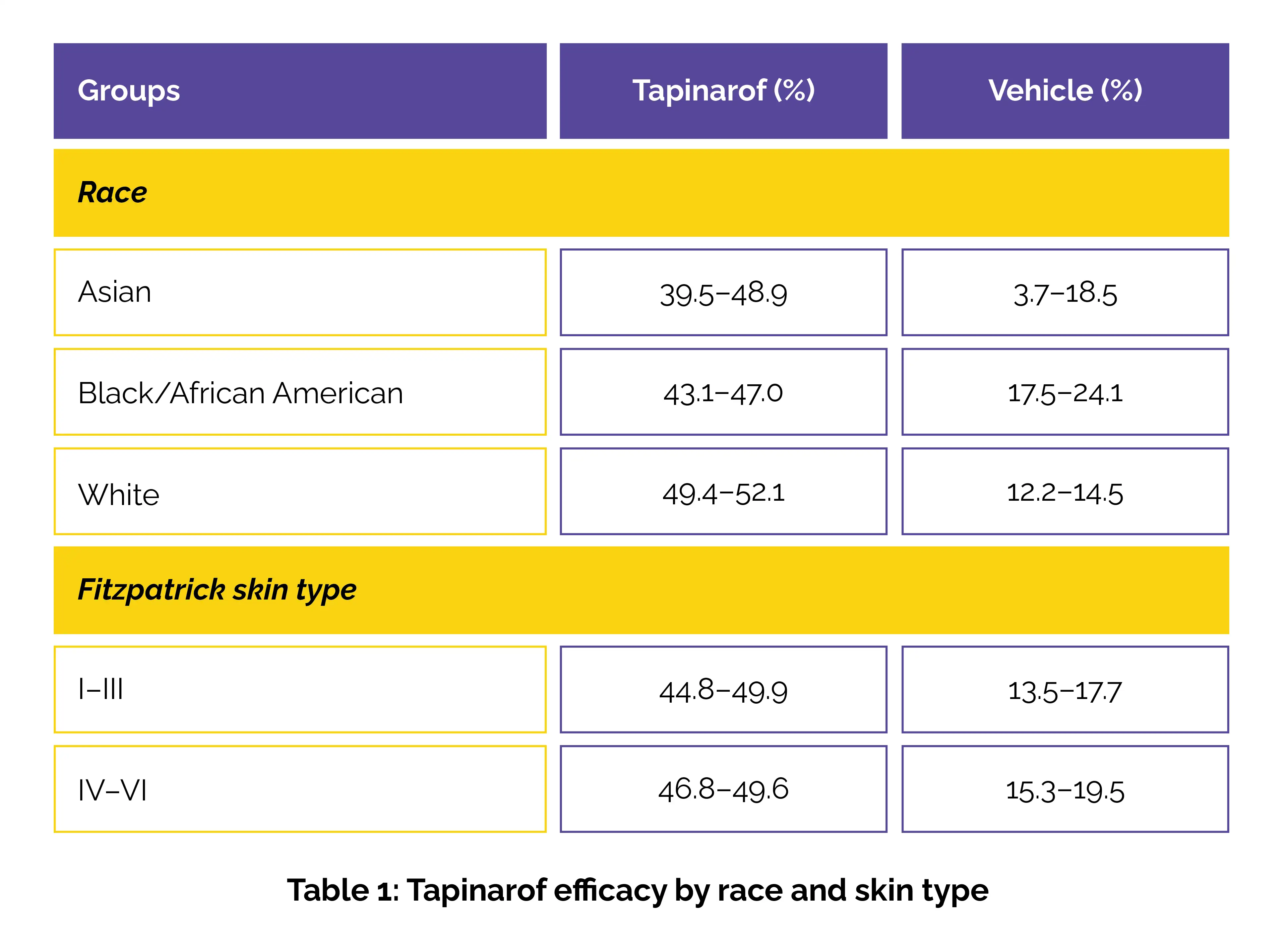
Treatment success was defined as a validated investigator global assessment for AD™ (vIGA-AD™ ) score of 0 (clear) or 1 (almost clear) with ≥2-grade improvement from baseline at week 8. Secondary outcomes included achieving ≥75% improvement in Eczema Area and Severity Index (EASI75). Efficacy was analyzed by race (Asian, Black/African American, white) and Fitzpatrick skin type (I–III, IV–VI) to assess performance across a diverse patient population.
In the ADORING 1 and 2 trials, 407 and 406 participants were randomized to receive either tapinarof or vehicle once daily. The racial distribution included 7.3–17.0% Asian, 25.9–35.1% Black/African American, and 43.0–57.7% White patients, with over half having Fitzpatrick skin types IV–VI. Tapinarof showed significant efficacy in both adults and children.
Across both trials, a greater proportion of participants treated with tapinarof achieved the primary endpoint compared with those receiving vehicle, regardless of race or Fitzpatrick skin type. Consistently superior responses were observed across all racial and skin type groups with tapinarof treatment (Table 1).
EASI75 responses were consistently greater with tapinarof compared to the vehicle. Most adverse events were mild-to-moderate, resulting in few discontinuations, which occurred less frequently with tapinarof than with the vehicle.
Topical use of 1% tapinarof cream delivered higher primary and EASI75 response rates than vehicle across all racial and skin type categories, underscoring its consistent therapeutic effect.
Dermatology and Therapy
Tapinarof Cream for Adults and Children with Atopic Dermatitis-Efficacy by Race and Fitzpatrick Skin Type in Two Phase 3 Randomized Clinical Trials
Andrew F Alexis et al.
Comments (0)