Categories
Change Password!
Reset Password!


Suppressive valacyclovir therapy does not reduce PHN prevalence but lowers pain severity, duration, and medication use in patients with herpes zoster ophthalmicus.
A new international clinical trial has found that while a year-long course of suppressive valacyclovir does not markedly reduce the overall prevalence of postherpetic neuralgia (PHN) in patients with herpes zoster ophthalmicus (HZO), it may offer notable benefits in minimizing pain severity and the requisition for neuropathic pain medications—particularly in younger individuals and those with chronic HZO. The multicenter, placebo-controlled randomized trial, involved 527 immunocompetent, nonpregnant adults across 95 sites in the United States, Canada, and New Zealand.
Eligible participants had a documented history of HZO rash with associated keratitis or iritis within the past year and a glomerular filtration rate of at least 45 mL/min/1.73 m². Volunteers were randomized to get either 1000 mg of oral valacyclovir daily or placebo for 12 months. Every 3 months, follow-up visits were executed. At the 12-month mark, the study found no pivotal difference in PHN prevalence between the valacyclovir group and placebo group (40%) (between-group difference, 2.5%). However, subgroup analysis revealed clinically meaningful secondary outcomes.
Among those younger than 60 years at HZO onset with chronic disease (≥6 months), those receiving valacyclovir reported markedly lower pain scores at both 12 months and 18 months. Furthermore, the duration of pain was found to be shorter in the valacyclovir group when compared to the placebo group (difference of -3.39 months). Valacyclovir use also led to reduced reliance on neuropathic pain medications. At 12 months, the mean daily dose was quite lower in the valacyclovir group than in the placebo group, a trend that continued at 18 months (Table 1).
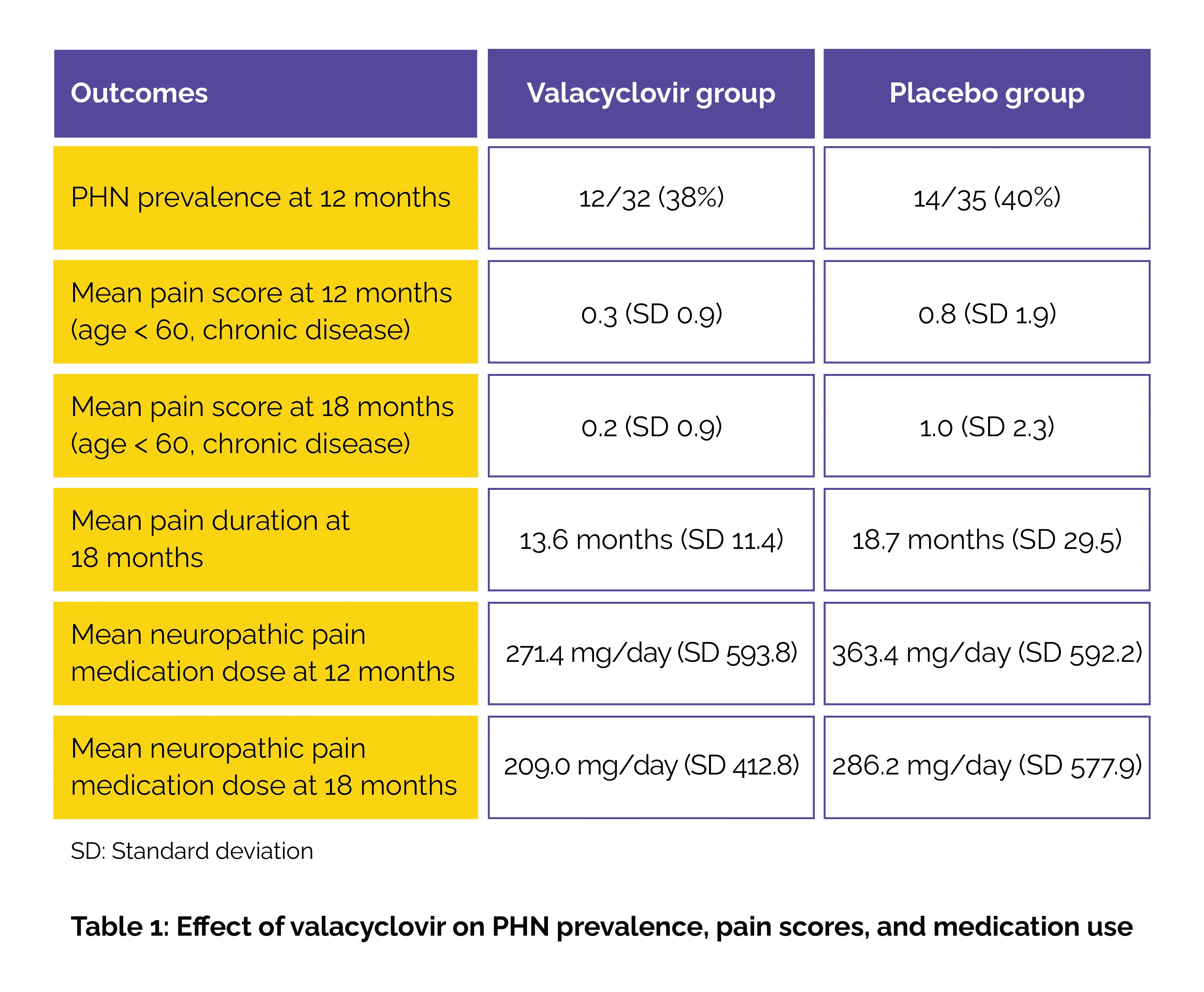
Overall, 490 participants completed the 12-month treatment, and 460 completed the full 18-month follow-up. Of the 73 who developed PHN, 59% were female, 10% were Hispanic, and 5% were Black or African American. The mean age was 62.4 years. While valacyclovir did not minimize the overall prevalence of PHN, researchers concluded that its impact on pain severity and medication use—especially in certain subgroups—supports considering 12-month suppressive therapy for selected patients with HZO.
JAMA Ophthalmology
Low-Dose Valacyclovir for Postherpetic Neuralgia in the Zoster Eye Disease Study: A Randomized Clinical Trial
David B Warner et al.
Comments (0)