Categories
Change Password!
Reset Password!


In NAFLD-affected people, dapagliflozin is effective and safe to lower CAP score and fatty liver grade, irrespective of their diabetes status.
In a trial led by Meng-Tzu Weng and other researchers, dapagliflozin demonstrated potential in reducing liver steatosis in individuals with moderate-to-severe nonalcoholic fatty liver disease (NAFLD). This study published in “Hepatology International” investigated the efficacy and safety of dapagliflozin (sodium-glucose cotransporter 2 [SGLT2] inhibitor primarily used for type 2 diabetes) for NAFLD management.
A total of 150 volunteers with NAFLD (including 20 with type 2 diabetes) and a controlled attenuation parameter (CAP) score of ≥252 dB/m were randomly segregated to either the dapagliflozin group or the control group. The key outcome was the alteration in CAP scores, estimated using FibroScan after 24 weeks of treatment.
In this randomized controlled trial, the dapagliflozin group experienced a remarkable reduction in CAP scores and fatty liver grade when compared to the control group. Though liver stiffness, waist circumference, and alanine transaminase (ALT) levels decreased in both groups, the differences were not statistically significant (Table 1).
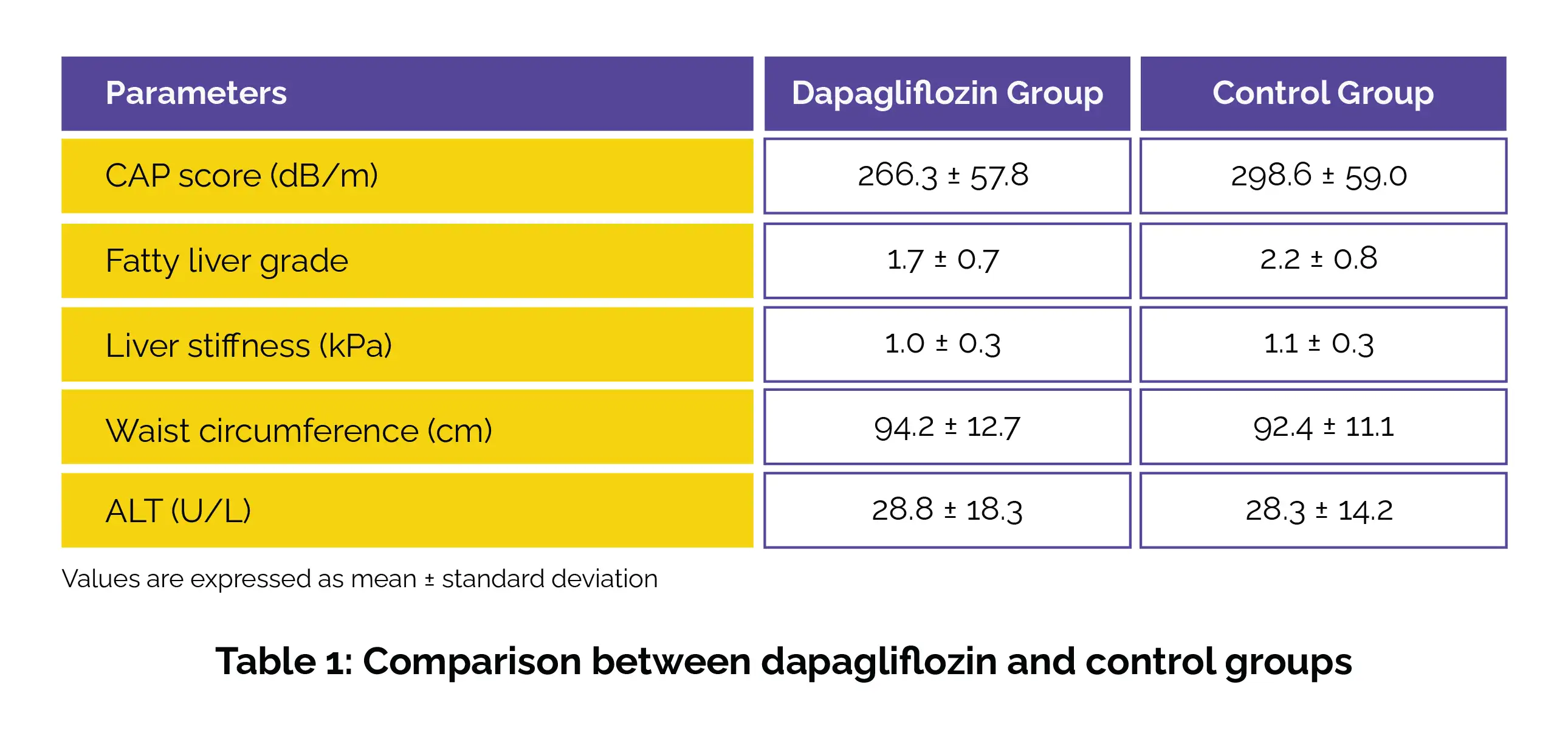
The multivariate analysis revealed that dapagliflozin treatment and changes in body mass index were associated with reduced CAP scores. No adverse events were reported, suggesting dapagliflozin's potential as a safe and reliable solution for improving liver health in NAFLD.
Hepatology International
Effects of dapagliflozin on liver steatosis in patients with nonalcoholic fatty liver disease: a randomized controlled trial
Meng-Tzu Weng et al.
Comments (0)