Categories
Change Password!
Reset Password!


Aprocitentan significantly lowers mean sitting and ambulatory blood pressure at low (10–12.5 mg) and medium (25 mg) doses, with no added benefit observed at higher doses (50 mg).
A new meta-analysis offers encouraging evidence that aprocitentan, a novel antihypertensive agent, successfully reduces blood pressure in patients with hypertension—particularly when administered at low-to-medium doses—without increasing the risk of adverse events.
An extensive literature search was carried out across PubMed, Embase, ClinicalTrials.gov, and the Cochrane Library to explore randomized controlled trials (RCTs) examining the efficacy and safety of aprocitentan versus placebo for hypertension. Based on the administered dose of aprocitentan, studies were categorized into three groups: low-dose (10–12.5 mg), medium-dose (25 mg), and high-dose (50 mg). Data from 5 RCTs involving a total of 1,224 participants demonstrated that aprocitentan competently lowered blood pressure at low and medium doses. |
Specifically, low-dose aprocitentan (10–12.5 mg) markedly reduced mean sitting systolic blood pressure (msSBP) and diastolic blood pressure (msDBP), while the medium dose (25 mg) achieved even greater reductions in msSBP and msDBP. Improvements were also observed in 24-hour ambulatory systolic and diastolic blood pressures (maSBP and maDBP), with both low and medium doses producing statistically significant declines. However, the high-dose group (50 mg) did not show a statistically significant difference in msSBP when compared to placebo, as shown in Table 1:
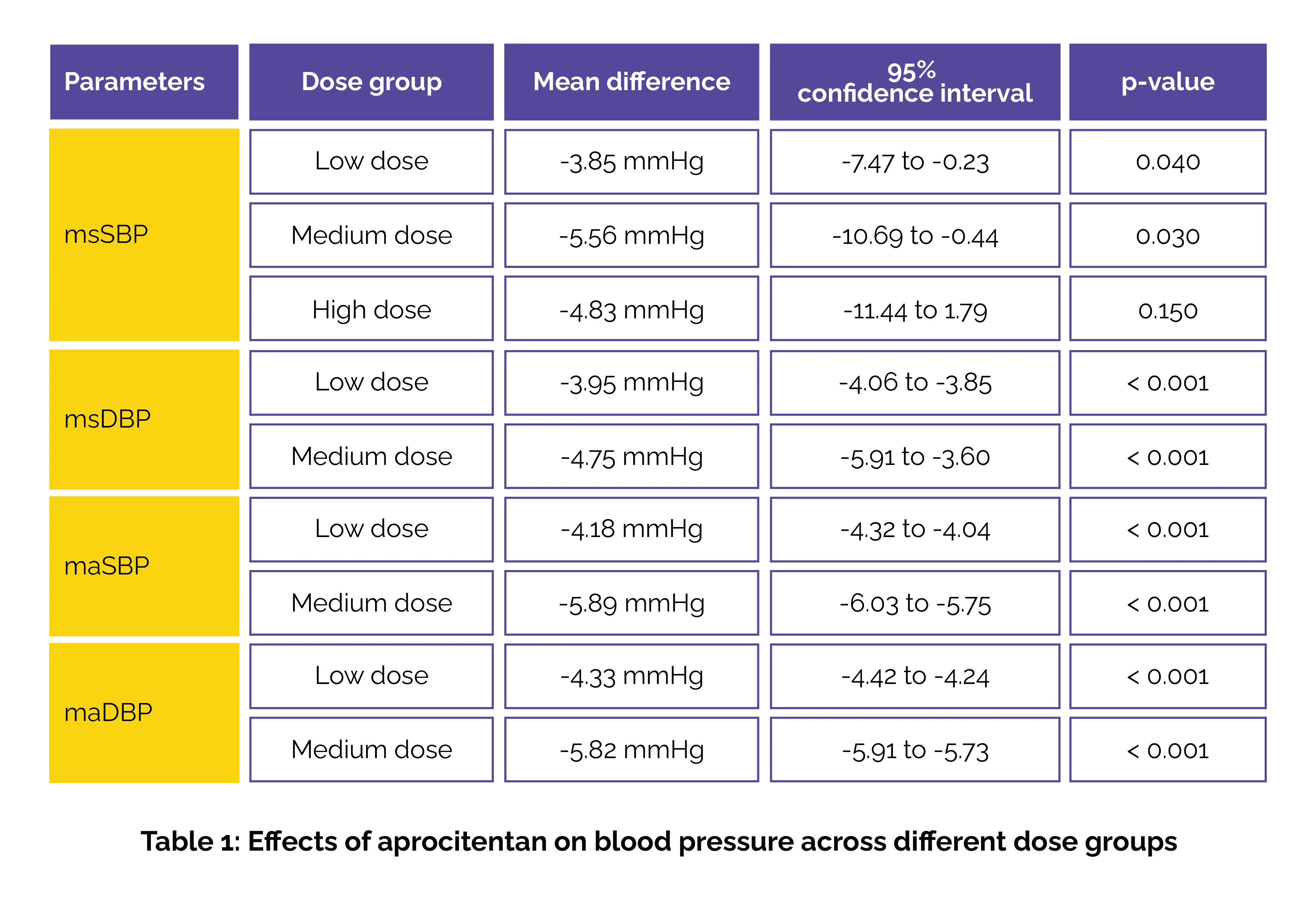
Across all dosing groups, aprocitentan was well-tolerated, with no considerable increase in adverse events or serious adverse events when compared to placebo. Thus, aprocitentan dramatically lowered blood pressure with a favorable safety profile, though higher doses (50 mg) did not provide additional benefits in blood pressure reduction.
Reviews in Cardiovascular Medicine
Efficacy and Safety of Aprocitentan in the Treatment of Hypertension: A Meta-Analysis of Evidence from Randomized Controlled Trials
Li Zheng et al.
Comments (0)