Categories
Change Password!
Reset Password!





Please verify that you are a Healthcare Professional. Register for complete access to all the content!

Oops...
There’s nothing here yet..


Tapinarof cream emerges as promising option for ad...
The findings of a study issued in "Archives of Dermatological Research" revealed the efficacy and safety of 1% Tapinarof cream in ameli...


Real-world study confirms efficacy of Cenobamate i...
In a real-world, observational study, the addition of Cenobamate as a treatment for refractory focal epilepsy demonstrated significant effectiven...
3 min

Study finds positive outcomes for nutritional inte...
In a revolutionary 16-week study newly published in “Arthritis & Rheumatology”, led by Robin Christensen and his team, an intensi...
2 min

VAS score ≥ 7 cm in endometriosis patients reflect...
A retrospective study conducted by Marina Paula Andres and other investigators revealed a strong association between visual analogue scale (VAS)...
2 min

Minoxidil-Spironolactone vs. Minoxidil-Finasteride...
A blinded randomized clinical trial aimed to evaluate the impact of topical Minoxidil plus oral Spironolactone, as opposed to the topical Minoxid...
3 min

Low-level laser therapy can ease heel lance pain i...
In a groundbreaking study, researchers have found that low-level laser therapy (LLLT) may offer comparable pain relief to breast milk during heel...
3 min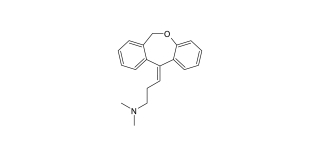

Doxepin
Doxepin is a dibenzoxepine derived-tricyclic antidepressant employed in the management of major depressive disorder (MDD), anxiety, and insomnia....
3 min

Study highlights safer alternatives to Valproate f...
A recent population-based cohort study has shed light on the comparative safety of antiseizure medication combinations during the first trimester...
3 min

Critical Insights into Diagnosis and Management of...
Infective endocarditis (IE) refers to inflammation affecting the endocardium (the heart's inner lining) and the valves that split the four ch...
9 min

Terbinafine is a preferred topical therapy for peo...
According to a study published in the ‘Diyala Journal of Medicine’, using Terbinafine twice a day for one week is equally effective i...
3 min

Prevalence and risk factors for chronic widespread...
Fibromyalgia is a common disorder characterized by widespread musculoskeletal pain. However, the exact cause of this prevalent condition is not e...
7 min

A cross-sectional study to explore the relationshi...
Chronic low back pain (CLBP) patients might suffer from sleep disorders. Adequate sleep hygiene is essential for the normal functioning of the bo...
6 min

Study determines whether antibiotic loaded bone ce...
This article aimed to perform a narrative review of recent literature to determine whether ALBC minimizes PJI risk in primary TKA.
6 min

Ketorolac trometamol: An effective preventive anal...
A study published in Stomatologiia depicted that preventive analgesia with ketorolac trometamol improves the efficacy of local anesthesia in the...
1 min

A case of COVID-19 pneumonia patient receiving con...
A 61-year old female was presented to the hospital with complaints of muscle pain and fever from the past four days. Her daughter was confirmed w...
5 min

Thank you for your participation!

An introduction to pain pathways and mechanisms
This video explains about pain and its different types, along with related mechanisms, pathways and receptors involved. It presents about nocicep...
9:10 min

Efficacy and safety of nonsteroidal anti-inflammat...
A number of NSAIDs are recommended for the treatment of pain and inflammation associated with knee osteoarthritis. In this study, the investigato...
5:50 min

Mode of action of rheumatoid arthritis
The video shows rheumatoid arthritis as a chronic and debilitating disease caused by a defect in one or many of the body's own defense mechanis...
1:10 min

Association between pain, central sensitization an...
Persistent nerve pain is one of the main complications of post herpetic neuralgia (PHN) but the mechanism causing pain is not really clear. In...
4:35 min

Nimesulide vs Ibuprofen to treat acute low back pa...
A randomized, prospective, double-blind, comparative trial was carried out to explore the efficacy and tolerability of nimesulide (a cyclooxygena...
4 min

Nimesulide vs Diclofenac to treat osteoarthritis o...
A double-blind, randomized, phase III clinical trial was conducted to compare nimesulide's safety and efficacy with diclofenac in patients suffer...
5 min

An introduction to pain pathways and mechanisms
This video explains about pain and its different types, along with related mechanisms, pathways and receptors involved. It presents about nocicep...
9:10 min

Thank you for your participation!


Reduced efficacy of sumatriptan in migraine with a...
A particular treatment does not produce same results in two different subtypes of a disease. This study has perfectly compared the efficacy of su...
5:05 min

Comparable analgesic efficacy of nimesulide and ke...
A non-randomized trial aimed to explore the efficacy of 100 mg nimesulide and 10 mg ketorolac to manage pain, swelling, and trismus after third m...
3 min

Improvement in efficacy of pancreatin in cystic fi...
In a double-blind, crossover fashion, this study aimed to explore the effect of adding Omeprazole to treatment with pancreatin on fecal fat excre...
3 min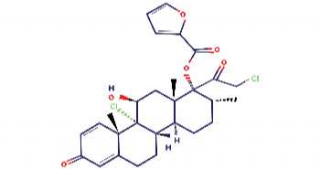

Mometasone furoate
Mometasone furoate is the furoate ester form of mometasone (a topical synthetic glucocorticoid receptor agonist).
5 min

Precision and Personalized Medicine: Unlocking the...
The field of medicine has witnessed incredible advancements in recent years, leading to a shift from a one-size-fits-all approach to a more perso...
14 min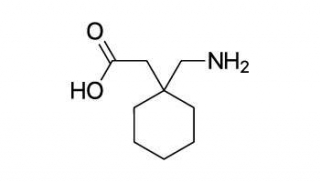

Gabapentin
Gabapentin is an anti-epileptic medication which is also known an anticonvulsant.
9 minThis website uses cookies to help us give you the best experience when you visit.
By using this website you consent to our use of cookies. For more information please review our cookie policy.

